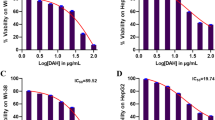
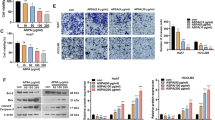
Abstract
Background
Hepatocellular carcinoma (HCC) is the most prevalent type of liver cancer and the main cause of cancer death globally. The use of medicinal herbs as chemotherapeutic agents in cancer treatment is receiving attention as they possess no or minimum side effects. Isorhamnetin (IRN), a flavonoid, has been under attention for its anti-inflammatory and anti-proliferative properties in a number of cancers, including colorectal, skin, and lung cancers. However, the in vivo mechanism of isorhamnetin to suppress liver cancer has yet to be explored.
Methods and Result
HCC was induced by N-diethylnitrosamine (DEN) and carbon tetrachloride (CCL4) in Swiss albino mice. Isorhamnetin (100 mg/kg body weight) was given to examine its anti-tumor properties in HCC mice model. Histological analysis and liver function assays were performed to assess changes in liver anatomy. Probable molecular pathways were explored using immunoblot, qPCR, ELISA, and immunohistochemistry techniques. Isorhamnetin inhibited various pro-inflammatory cytokines to suppress cancer-inducing inflammation. Additionally, it regulated Akt and MAPKs to suppress Nrf2 signaling. Isorhamnetin activated PPAR-γ and autophagy while suppressing cell cycle progression in DEN + CCl4-administered mice. Additionally, isorhamnetin regulated various signaling pathways to suppress cell proliferation, metabolism, and epithelial–mesenchymal transition in HCC.
Conclusion
Regulating diverse cellular signaling pathways makes isorhamnetin a better anti-cancer chemotherapeutic candidate in HCC. Importantly, the anti-TNF-α properties of isorhamnetin could prove it a valuable therapeutic agent in sorafenib-resistant HCC patients. Additionally, anti-TGF-β properties of isorhamnetin could be utilized to reduce the EMT-inducing side effects of doxorubicin.






Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- ATG7:
-
Autophagy-related 7
- Bad:
-
BCL2-associated agonist of cell death
- CCl4 :
-
Carbon tetrachloride
- C/EBP-δ:
-
CCAAT/enhancer-binding protein delta
- DEN:
-
N-Diethylnitrosamine
- Doxo:
-
Doxorubicin
- ERK:
-
Extracellular signal-regulated kinase
- EMT:
-
Epithelial–mesenchymal transition
- GSK-3β:
-
Glycogen synthase kinase-3 beta:
- HCC:
-
Hepatocellular carcinoma
- HO-1:
-
Heme Oxygenase-1
- HRP:
-
Horseradish peroxidise
- IRN:
-
Isorhamnetin
- JNK:
-
C-Jun N-terminal kinase
- Keap1:
-
Kelch-like ECH-associated protein 1
- Lamp2A:
-
Lysosomal associated membrane protein-2
- MMP-9:
-
Matrix metalloproteinases 9
- Mcl-1:
-
Myeloid cell leukemia-1
- mTOR:
-
Mammalian target of rapamycin
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- NSCLC:
-
Non-small cell lung carcinoma
- PPAR-γ:
-
Peroxisome proliferator-activated receptor-gamma
- ROS:
-
Reactive oxygen species
- RSK:
-
Ribosomal S6 kinase
- RUNX2:
-
Runt-related transcription factor 2
- SOD:
-
Superoxide dismutase
- SDS:
-
Sodium dodecyl sulfate
- SVR:
-
Sustained virological response
- STAT3:
-
Signal transducer and activator of transcription 3
- TGF-β:
-
Transforming growth factor beta
- TNF-α:
-
Tumor necrosis factor alpha
- YAP1:
-
Yes-associated protein 1
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Chavda HJ. Hepatocellular carcinoma in India. Indian J Surg. 2021;83(4):959–66.
Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380(15):1450–62.
Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15(10):599–616.
Ali Abdalla YO, Subramaniam B, Nyamathulla S, Shamsuddin, et al. Natural products for cancer therapy: a review of their mechanism of actions and toxicity in the past decade. J Trop Med. 2022;2022: e5794350.
Ghosh N, Kundu M, Ghosh S, Das AK, De S, Das J, Sil PC. pH-responsive and targeted delivery of chrysin via folic acid-functionalized mesoporous silica nanocarrier for breast cancer therapy. Int J Pharm. 2023;631: 122555.
Bhattacharya D, Sinha R, Mukherjee P, et al. Anti-virulence activity of polyphenolic fraction isolated from Kombucha against Vibrio cholerae. Microb Pathog. 2020;140: 103927.
Gong G, Guan YY, Zhang ZL, Rahman K, et al. Isorhamnetin: a review of pharmacological effects. Biomed Pharmacother. 2020;128: 110301.
Antunes-Ricardo M, Moreno-García BE, Gutiérrez-Uribe JA, et al. Induction of apoptosis in colon cancer cells treated with isorhamnetin glycosides from Opuntia ficus-indica pads. Plant Foods Hum Nutr. 2014;69:331–6.
Hu S, Huang L, Meng L, et al. Isorhamnetin inhibits cell proliferation and induces apoptosis in breast cancer via Akt and mitogen-activated protein kinase kinase signaling pathways. Mol Med Rep. 2015;12(5):6745–51.
Li Y, Fan B, Pu N, et al. Isorhamnetin suppresses human gastric cancer cell proliferation through mitochondria-dependent apoptosis. Molecules. 2022;27(16):5191.
Zhang BY, Wang YM, Gong H, et al. Isorhamnetin flavonoid synergistically enhances the anticancer activity and apoptosis induction by cis-platin and carboplatin in non-small cell lung carcinoma (NSCLC). Int J Clin Exp Pathol. 2015;8(1):25–37.
Lu X, Liu T, Chen K, et al. Isorhamnetin: a hepatoprotective flavonoid inhibits apoptosis and autophagy via P38/PPAR-α pathway in mice. Biomed Pharmacother. 2018;103:800–11.
Liu N, Feng J, Lu X, et al. Isorhamnetin inhibits liver fibrosis by reducing autophagy and inhibiting extracellular matrix formation via the TGF-β1/Smad3 and TGF-β1/p38 MAPK pathways. Mediat Inflamm. 2019;2019:1–14.
Yang JH, Shin BY, Han JY, Kim MG, Wi JE, Kim YW, et al. Isorhamnetin protects against oxidative stress by activating Nrf2 and inducing the expression of its target genes. Toxicol Appl Pharmacol. 2014;274(2):293–301.
Uehara T, Pogribny IP, Rusyn I. The DEN and CCl4 -induced mouse model of fibrosis and inflammation-associated hepatocellular carcinoma. Curr Protoc Pharmacol. 2014;66:14–30.
Sur S, Pal D, Mandal S, Roy A, Panda CK. Tea polyphenols epigallocatechin gallete and theaflavin restrict mouse liver carcinogenesis through modulation of self-renewal Wnt and hedgehog pathways. J Nutr Biochem. 2016;27:32–42.
Fei R, Wei H. Quantitative proteomic analysis of Isorhamnetin treatment in human liver cancer cells. J Med Plants. 2018;12(7):77–88.
Teng BS, Lu YH, Wang ZT, et al. In vitro anti-tumor activity of isorhamnetin isolated from Hippophae rhamnoides L. against BEL-7402 cells. Pharmacol Res. 2006;54(3):186–94.
Park C, Cha HJ, Choi EO, et al. Isorhamnetin induces cell cycle arrest and apoptosis via reactive oxygen species-mediated AMP-activated protein kinase signaling pathway activation in human bladder cancer cells. Cancers. 2019;11(10):1494.
Cai F, Zhang Y, Li J, et al. Isorhamnetin inhibited the proliferation and metastasis of androgen-independent prostate cancer cells by targeting the mitochondrion-dependent intrinsic apoptotic and PI3K/Akt/mTOR pathway. Biosci Rep. 2020;40(3):BSR20192826.
Ramachandran L, Manu KA, Shanmugam MK, et al. Isorhamnetin inhibits proliferation and invasion and induces apoptosis through the modulation of peroxisome proliferator-activated receptor γ activation pathway in gastric cancer. J Biol Chem. 2012;287(45):38028–40.
Sun J, Sun G, Meng X, et al. Isorhamnetin protects against doxorubicin-induced cardiotoxicity in vivo and in vitro. PLoS ONE. 2013;8(5): e64526.
Jin C, Li H, He Y, et al. Combination chemotherapy of doxorubicin and paclitaxel for hepatocellular carcinoma in vitro and in vivo. J Cancer Res Clin Oncol. 2010;136:267–74.
Manna P, Sinha M, Sil PC. Protection of arsenic-induced hepatic disorder by arjunolic acid. Basic Clin Pharmacol Toxicol. 2007;101(5):333–8.
Chowdhury S, Ghosh S, Rashid K, Sil PC. Deciphering the role of ferulic acid against streptozotocin-induced cellular stress in the cardiac tissue of diabetic rats. Food Chem Toxicol. 2016;97:187–98.
Das AK, Hossain U, Ghosh S, et al. Amelioration of oxidative stress mediated inflammation and apoptosis in pancreatic islets by Lupeol in STZ-induced hyperglycaemic mice. Life Sci. 2022;305: 120769.
Manna P, Sinha M, Sil PC. Prophylactic role of arjunolic acid in response to streptozotocin mediated diabetic renal injury: activation of polyol pathway and oxidative stress responsive signaling cascades. Chem Biol Interact. 2009;181(3):297–308.
Manna P, Ghosh J, Das J, Sil PC. Streptozotocin induced activation of oxidative stress responsive splenic cell signaling pathways: protective role of arjunolic acid. Toxicol Appl Pharmacol. 2010;244(2):114–29.
Saha S, Sadhukhan P, Sinha K, Agarwal N, Sil PC. Mangiferin attenuates oxidative stress induced renal cell damage through activation of PI3K induced Akt and Nrf-2 mediated signaling pathways. Biochem Biophys Rep. 2016;5:313–27.
Ghosh N, Chatterjee S, Biswal D, Pramanik NR, Chakrabarti S, Sil PC. Oxidative stress imposed in vivo anticancer therapeutic efficacy of novel imidazole-based oxidovanadium (IV) complex in solid tumor. Life Sci. 2022;301: 120606.
Ambade A, Satishchandran A, Gyongyosi B, et al. Adult mouse model of early hepatocellular carcinoma promoted by alcoholic liver disease. World J Gastroenterol. 2016;22(16):4091.
Fujiwara M, Kwok S, Yano H, Pai RK. Arginase-1 is a more sensitive marker of hepatic differentiation than HepPar-1 and glypican-3 in fine-needle aspiration biopsies. Cancer Cytopathol. 2012;120(4):230–7.
Radwan NA, Ahmed NS. The diagnostic value of arginase-1 immunostaining in differentiating hepatocellular carcinoma from metastatic carcinoma and cholangiocarcinoma as compared to HepPar-1. Diagnostic Pathol. 2012;7:1–12.
Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6.
Li W, Jian YB. Antitumor necrosis factor-alpha antibodies as a noveltherapy for hepatocellular carcinoma. Exp Ther Med. 2018;16(2):529–36.
Das M, Sabio G, Jiang F, et al. Induction of hepatitis by JNK-mediated expression of TNF-α. Cell. 2009;136(2):249–60.
Eferl R, Ricci R, Kenner L, et al. Liver tumor development: c-Jun antagonizes the proapoptotic activity of p53. Cell. 2003;112(2):181–92.
Kudo M, Sugawara A, Uruno A, et al. Transcription suppression of peroxisome proliferator-activated receptor γ2 gene expression by tumor necrosis factor α via an inhibition of CCAAT/enhancer-binding protein δ during the early stage of adipocyte differentiation. Endocrinology. 2004;145(11):4948–56.
Adams M, Reginato MJ, Shao D, et al. Transcriptional activation by peroxisome proliferator-activated receptor gamma is inhibited by phosphorylation at a consensus mitogen-activated protein kinase site. J Biol Chem. 1997;272(8):5128–32.
Hsu HT, Chi CW. Emerging role of the peroxisome proliferator-activated receptor-gamma in hepatocellular carcinoma. J Hepatocell Carcinoma. 2014;1:127–35.
Scheau C, Badarau IA, Costache R, et al. The role of matrix metalloproteinases in the epithelial-mesenchymal transition of hepatocellular carcinoma. Anal Cell Pathol. 2019;2019:9423907.
Shen B, Chu ES, Zhao G, et al. PPARgamma inhibits hepatocellular carcinoma metastases in vitro and in mice. Br J Cancer. 2012;106(9):1486–94.
Sarkar S, Ghosh N, Kundu M, Sil PC (2020) Nrf2 and Inflammation-Triggered Carcinogenesis. In: Deng H (eds) Nrf2 and its Modulation in Inflammation. Progress in Inflammation Research, vol 85th. Springer, Cham, pp 129–152.
Doehn U, Hauge C, Frank SR, et al. RSK is a principal effector of the RAS-ERK pathway for eliciting a coordinate promotile/invasive gene program and phenotype in epithelial cells. Mol Cell. 2009;35(4):511–22.
Tong J, Wang P, Tan S, et al. Mcl-1 degradation is required for targeted therapeutics to eradicate colon cancer cells. Cancer Res. 2017;77(9):2512–21.
Deane NG, Parker MA, Aramandla R, et al. Hepatocellular carcinoma results from chronic cyclin D1 overexpression in transgenic mice. Cancer Res. 2001;61(14):5389–95.
Kossatz U, Malek NP. p27: tumor suppressor and oncogene …? Cell Res. 2007;17(10):832–3.
Wagayama H, Shiraki K, Sugimoto K, et al. High expression of p21WAF1/CIP1 is correlated with human hepatocellular carcinoma in patients with hepatitis C virus-associated chronic liver diseases. Hum Pathol. 2002;33(4):429–34.
Shiraki K, Wagayama H. Cytoplasmic p21(WAF1/CIP1) expression in human hepatocellular carcinomas. Liver Int. 2006;26(8):1018–9.
Das AK, Ghosh N, Mandal A, Sil PC. Glycobiology of cellular expiry: decrypting the role of glycan-lectin regulatory complex and therapeutic strategies focusing on cancer. Biochem Pharmacol. 2022;207: 115367.
Lee YA, Noon LA, Akat KM, et al. Autophagy is a gatekeeper of hepatic differentiation and carcinogenesis by controlling the degradation of Yap. Nat Commun. 2018;9(1):4962.
Ghosh N, Hossain U, Mandal A, Sil PC. The Wnt signaling pathway: a potential therapeutic target against cancer. Ann N Y Acad Sci. 2019;1443(1):54–74.
Tan W, Luo X, Li W, et al. TNF-alpha is a potential therapeutic target to overcome sorafenib resistance in hepatocellular carcinoma. EBioMedicine. 2019;40:446–56.
Ye J. Regulation of PPARgamma function by TNF-alpha. Biochem Biophys Res Commun. 2008;374(3):405–8.
Acknowledgements
The authors are deeply grateful to Ms. Noyel Ghosh and Ms. Ankita Mandal for their valuable inputs during the preparation of the manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
SS contributed to conceptualization, methodology, software, validation, data curation, formal analysis, investigation, writing of the original draft, visualization, and writing, reviewing, & editing of the manuscript. AKD contributed to methodology, validation, and writing, reviewing, & editing of the manuscript. SB contributed to conceptualization. RG contributed to conceptualization, validation, data curation, formal analysis, visualization, and writing, reviewing, & editing of the manuscript. PCS contributed to conceptualization, validation, data curation, formal analysis, visualization, and writing, reviewing, & editing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
All the animal experiments were prosecuted according to the institutional ethical committee and with the permission of IAEC, CPCSEA (Committee for the Purpose of Control & Supervision on Experiments on Animals) and the Ministry of Environment and Forests, New Delhi, India [1796/GO/EReBiBt/S/14/CPCSEA].
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sarkar, S., Das, A.K., Bhattacharya, S. et al. Isorhamnetin exerts anti-tumor activity in DEN + CCl4-induced HCC mice. Med Oncol 40, 188 (2023). https://doi.org/10.1007/s12032-023-02050-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02050-5