Abstract
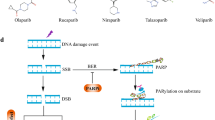
When DNA repair is inadequate it increases the chances of the genome becoming unstable and it undergoes a malignant mutation. The deficiency of DNA repair PARP proteins may be leveraged for cancer therapy by increasing genomic instability and causing massive DNA damage in cancer cells. DNA repair components are under increased demand in cancer cells because of the continuous replication of DNA. The oncogenic loss of BRCA and an inefficient DNA repair led to cancer cells being dependent on particular DNA repair pathways, like the Poly (ADP-ribose) polymerase pathway. Breast cancer gene 1 and 2 plays a crucial role in DNA repair and genome integrity explaining how BRCA1 and BRCA2 mutations raise the menace of cancer. PARP inhibitors inhibit the base exclusion repair pathway, resulting in the buildup of unrepaired single strand breaks, which cause inflated replication forks in the S phase and subsequently the development of damaging double stranded breaks. Cells having BRCA mutations are unable to repair DNA breaks, leading to apoptosis and eventually death of cancer cells. Numerous indicators, such as a lack of homologous recombination and a high degree of replication pressure, indicate that this therapy will be very effective. Combining PARP inhibitors with chemotherapy, an immune checkpoint inhibitor, and a targeted drug is an effective strategy for combating PARP inhibitors resistance. Several PARP-based combination approaches are in preclinical and clinical development. Various clinical trials are successfully completed and some are undergoing to evaluate the efficacy of these molecules. This review will describe the current views and clinical updates on PARP inhibitors.
Graphical abstract






Similar content being viewed by others
Data availability
This submission does not require any availability of data and materials as this is a review paper.
References
Rosen EM, Fan S, Pestell RG, Goldberg ID. BRCA1 gene in breast cancer. J Cell Physiol. 2003;196(1):19–41. https://doi.org/10.1002/jcp.10257.
Godet I, Gilkes DM. BRCA1 and BRCA2 mutations and treatment strategies for breast cancer. Integr Cancer Sci Ther. 2017. https://doi.org/10.15761/ICST.1000228.
Moschetta M, George A, Kaye SB, Banerjee S. BRCA somatic mutations and epigenetic BRCA modifications in serous ovarian cancer. Ann Oncol. 2016;27(8):1449–55. https://doi.org/10.1093/annonc/mdw142.
Dziadkowiec KN, Gąsiorowska E, Nowak-Markwitz E, Jankowska A. PARP inhibitors: review of mechanisms of action and brca1/2 mutation targeting. Menopausal Rev. 2016;4:215–9. https://doi.org/10.5114/pm.2016.65667.
Mateo J, Lord CJ, Serra V, Tutt A, Balmaña J, Castroviejo-Bermejo M, Cruz C, Oaknin A, Kaye SB, de Bono JS. A decade of clinical development of PARP inhibitors in perspective. Ann Oncol. 2019;30(9):1437–47. https://doi.org/10.1093/annonc/mdz192.
Welcsh PL, Owens KN, King M-C. Insights into the functions of BRCA1 and BRCA2. Trends Genet. 2000;16(2):69–74. https://doi.org/10.1016/S0168-9525(99)01930-7.
Jasin M. Homologous repair of DNA damage and tumorigenesis: the BRCA connection. Oncogene. 2002;21(58):8981–93. https://doi.org/10.1038/sj.onc.1206176.
Roy R, Chun J, Powell SN. BRCA1 and BRCA2: different roles in a common pathway of genome protection. Nat Rev Cancer. 2012;12(1):68–78. https://doi.org/10.1038/nrc3181.
Flippot R, Patrikidou A, Aldea M, Colomba E, Lavaud P, Albigès L, Naoun N, Blanchard P, Terlizzi M, Garcia C, Bernard-Tessier A, Fuerea A, Di Palma M, Escudier B, Loriot Y, Baciarello G, Fizazi K. PARP Inhibition, a new therapeutic avenue in patients with prostate cancer. Drugs. 2022. https://doi.org/10.1007/s40265-022-01703-5.
Sonnenblick A, de Azambuja E, Azim HA, Piccart M. An update on PARP inhibitors: moving to the adjuvant setting. Nat Rev Clin Oncol. 2015;12(1):27–41. https://doi.org/10.1038/nrclinonc.2014.163.
Herceg Z, Wang Z-Q. Functions of poly (ADP-ribose) polymerase (PARP) in DNA repair, genomic integrity and cell death. Mutat Res/Fundam Mol Mech Mutagenesis. 2001;477(1–2):97–110. https://doi.org/10.1016/S0027-5107(01)00111-7.
Arun B, Akar U, Gutierrez-Barrera AM, Hortobagyi GN, Ozpolat B. The PARP inhibitor AZD2281 (Olaparib) induces autophagy/mitophagy in BRCA1 and BRCA2 mutant breast cancer cells. Int J Oncol. 2015;47(1):262–8. https://doi.org/10.3892/ijo.2015.3003.
Wang Z, Wang F, Tang T, Guo C. The role of PARP1 in the DNA damage response and its application in tumor therapy. Front Med. 2012;6(2):156–64. https://doi.org/10.1007/s11684-012-0197-3.
Jeggo PA. DNA repair: PARP – another guardian angel? Curr Biol. 1998;8(2):R49–51. https://doi.org/10.1016/S0960-9822(98)70032-6.
Bochum S, Berger S, Martens UM. Olaparib. Small Mol Oncol. 2018. https://doi.org/10.1007/978-3-319-91442-8_15.
Krokan HE, Bjoras M. Base excision repair. Cold Spring Harb Perspect Biol. 2013;5(4):a012583. https://doi.org/10.1101/cshperspect.a012583.
Ceccaldi R, Rondinelli B, D’Andrea AD. Repair pathway choices and consequences at the double-strand break. Trends Cell Biol. 2016;26(1):52–64. https://doi.org/10.1016/j.tcb.2015.07.009.
Farmer H, McCabe N, Lord CJ, Tutt ANJ, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, Martin NMB, Jackson SP, Smith GCM, Ashworth A. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–21. https://doi.org/10.1038/nature03445.
de Murcia G, de Murcia JM. Poly(ADP-ribose) polymerase: a molecular nick-sensor. Trends Biochem Sci. 1994;19(4):172–6. https://doi.org/10.1016/0968-0004(94)90280-1.
Wang L, Liang C, Li F, Guan D, Wu X, Fu X, Lu A, Zhang G. PARP1 in carcinomas and PARP1 inhibitors as antineoplastic drugs. Int J Mol Sci. 2017;18(10):2111. https://doi.org/10.3390/ijms18102111.
Abbotts R, Dellomo AJ, Rassool FV. Pharmacologic induction of BRCAness in BRCA-proficient cancers: expanding PARP inhibitor use. Cancers. 2022;14(11):2640. https://doi.org/10.3390/cancers14112640.
Research, C. for D. E. FDA approves olaparib for adjuvant treatment of high-risk early breast cancer. FDA. 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-olaparib-adjuvant-treatment-high-risk-early-breast-cancer.
LYNPARZA® (Olaparib). (n.d.). https://www.lynparza.com.
Rubraca® (rucaparib) tablets | Rubraca® (rucaparib) tablets. (n.d.). https://www.rubraca.com/.
Rucaparib | FDA. (n.d.). https://www.fda.gov/drugs/resources-information-approved-drugs/rucaparib.
Biopharma TE. Rucaparib cost and price in India, used for ovarian cancer. Elevation Biopharma. 2021. https://elevationbiopharma.com/rucaparib-cost-and-price-in-delhi-india/.
Research, C. for D. E. FDA approves niraparib for first-line maintenance of advanced ovarian cancer. FDA. 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-niraparib-first-line-maintenance-advanced-ovarian-cancer.
Mashimo M, Kita M, Uno A, Nii M, Ishihara M, Honda T, Gotoh-Kinoshita Y, Nomura A, Nakamura H, Murayama T, Kizu R, Fujii T. Tankyrase regulates neurite outgrowth through poly(ADP-ribosyl)ation-dependent activation of β-catenin signaling. Int J Mol Sci. 2022;23(5):2834. https://doi.org/10.3390/ijms23052834.
Haikarainen T, Krauss S, Lehtio L. Tankyrases: structure, function and therapeutic implications in cancer. Curr Pharm Des. 2014;20(41):6472–88. https://doi.org/10.2174/1381612820666140630101525.
Riffell JL, Lord CJ, Ashworth A. Tankyrase-targeted therapeutics: expanding opportunities in the PARP family. Nat Rev Drug Discov. 2012;11(12):923–36. https://doi.org/10.1038/nrd3868.
Allarity therapeutics. Phase II, open label clinical study to investigate anti-tumour effect and tolerability of the PARP inhibitor 2X-121 in patients with metastatic breast cancer selected by the 2X-121 DRP. clinicaltrials.gov. 2022. https://clinicaltrials.gov/ct2/show/study/NCT03562832. Accessed 10 May 2022.
New Mexico Cancer Care Alliance. A phase 1-2 study of the combination of Olaparib and Tremelimumab, in BRCA1 and BRCA2 mutation carriers With recurrent ovarian cancer (Clinical Trial Registration No. NCT02571725). clinicaltrials.gov. 2022. https://clinicaltrials.gov/ct2/show/NCT02571725.
Kroep JR. Durvalumab and Olaparib in metastatic or recurrent endometrial cancer (Clinical Trial Registration No. NCT03951415). clinicaltrials.gov. 2021. https://clinicaltrials.gov/ct2/show/NCT03951415
MD, AC. Phase I study of veliparib (ABT-888), an Oral PARP inhibitor, and VX-970, an ATR inhibitor in combination with cisplatin in patients with refractory solid tumors (Clinical Trial Registration No. NCT02723864). clinicaltrials.gov. 2022. https://clinicaltrials.gov/ct2/show/NCT02723864 .
de Bono J, Ramanathan RK, Mina L, et al. Phase I, dose-escalation, two-part trial of the PARP inhibitor talazoparib in patients with advanced germline BRCA1/2 mutations and selected sporadic cancers. Cancer Discov. 2017;7(6):620–9. https://doi.org/10.1158/2159-8290.CD-16-1250.
Tesaro Inc. A phase 2, single-arm, open-label study to evaluate the safety and efficacy of niraparib combined with bevacizumab as maintenance treatment in patients with advanced ovarian cancer, fallopian tube cancer, or primary peritoneal cancer following front-line platinum-based chemotherapy with bevacizumab (Clinical Trial Registration No. NCT03326193). clinicaltrials.gov. 2021. https://clinicaltrials.gov/ct2/show/NCT03326193.
M.D. Anderson Cancer Center. A phase Ib study of the oral PARP inhibitor Olaparib with the oral mTORC1/2 inhibitor AZD2014 or the oral AKT inhibitor AZD5363 for recurrent endometrial, triple negative breast, and ovarian, primary peritoneal, or fallopian tube cancer (Clinical Trial Registration No. NCT02208375). clinicaltrials.gov. 2022. https://clinicaltrials.gov/ct2/show/NCT02208375.
University of Chicago. Phase II trial of Olaparib in homologous recombination deficient (HRD) malignant mesothelioma (Clinical Trial Registration No. NCT04515836). clinicaltrials.gov. 2021. https://clinicaltrials.gov/ct2/show/NCT04515836.
Sanai N. A phase 0/2 clinical trial of pamiparib in newly–diagnosed and recurrent glioblastoma patients (Clinical Trial Registration No. NCT04614909). clinicaltrials.gov. 2022. https://clinicaltrials.gov/ct2/show/NCT04614909.
Peter MacCallum Cancer Centre, Australia. Phase 1 trial of PARP inhibitor combined with 177Lu-DOTA-octreotate peptide receptor radionuclide therapy (PRRT) in patients with metastatic neuroendocrine tumor (Clinical Trial Registration No. NCT05053854). clinicaltrials.gov. 2021. https://clinicaltrials.gov/ct2/show/NCT05053854.
Gruppo Oncologico del Nord-Ovest. Induction and maintenance treatment with PARP inhibitor and immunotherapy in HPV-negative head and neck squamous cell carcinoma (HNSCC) (Clinical Trial Registration No. NCT04681469). clinicaltrials.gov. 2021. https://clinicaltrials.gov/ct2/show/NCT04681469.
Ellipses Pharma. Phase 2 study to evaluate the safety & efficacy of EP0057 in combination with Olaparib in advanced ovarian cancer patients who have: cohort 1—platinum resistant disease; cohort 2—had at least 1 prior line of therapy which must include at least 1 line of platinum-BASED chemotherapy followed by PARP inhibitor maintenance (Clinical Trial Registration No. NCT04669002). clinicaltrials.gov. 2022. https://clinicaltrials.gov/ct2/show/NCT04669002.
University of Michigan Rogel Cancer Center. Phase II multi-center study of PARP inhibitor rucaparib in combination with anti-PD-1 antibody nivolumab in patients with advanced or metastatic biliary tract cancer following platinum therapy (Clinical Trial Registration No. NCT03639935). clinicaltrials.gov. 2022. https://clinicaltrials.gov/ct2/show/NCT03639935.
Jonsson Comprehensive Cancer Center. A phase 2 study of pamiparib (BGB-290) plus temozolomide for hereditary leiomyomatosis and renal cell cancer (HLRCC) (Clinical Trial Registration No. NCT04603365). clinicaltrials.gov. 2021. https://clinicaltrials.gov/ct2/show/NCT04603365.
Sandhu D, Antolin AA, Cox AR, Jones AM. Identification of different side effects between PARP inhibitors and their polypharmacological multi-target rationale. Br J Clin Pharmacol. 2022;88(2):742–52. https://doi.org/10.1111/bcp.15015.
Antolin AA, Ameratunga M, Banerji U, Clarke PA, Workman P, Al-Lazikani B. The kinase polypharmacology landscape of clinical PARP inhibitors. Sci Rep. 2020;10(1):2585. https://doi.org/10.1038/s41598-020-59074-4.
Tian X, Chen L, Gai D, He S, Jiang X, Zhang N. Adverse event profiles of PARP inhibitors: analysis of spontaneous reports submitted to FAERS. Front Pharmacol. 2022;13: 851246. https://doi.org/10.3389/fphar.2022.851246.
Zhou JX, Feng LJ, Zhang X. Risk of severe hematologic toxicities in cancer patients treated with PARP inhibitors: a meta-analysis of randomized controlled trials. Drug Des Dev Ther. 2017;11:3009–17. https://doi.org/10.2147/DDDT.S147726.
Research, C. for D. E. FDA Adverse Event Reporting System (FAERS) Public Dashboard. FDA. 2021. https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard.
Master SR, Mansour RP. Myelodysplastic syndrome and acute myeloid leukemia as side effect of PARP inhibitors. J Clin Oncol. 2020. https://doi.org/10.1200/JCO.2020.38.15_suppl.3601.
Nitecki R, Melamed A, Gockley AA, Floyd J, Krause KJ, Coleman RL, Matulonis UA, Giordano SH, Lu KH, Rauh-Hain JA. Incidence of myelodysplastic syndrome and acute myeloid leukemia in patients receiving poly-ADP ribose polymerase inhibitors for the treatment of solid tumors: a meta-analysis of randomized trials. Gynecol Oncol. 2021;161(3):653–9. https://doi.org/10.1016/j.ygyno.2021.03.011.
Sun J, Liu J, Gao C, Zheng J, Zhang J, Ding Y, Gong W, Yang M, Li Z, Wang Y, Yang Y, Gao C. Targeted delivery of PARP inhibitors to neuronal mitochondria via biomimetic engineered nanosystems in a mouse model of traumatic brain injury. Acta Biomater. 2022;140:573–85. https://doi.org/10.1016/j.actbio.2021.12.023.
Guney Eskiler G, Ozturk M. Therapeutic potential of the PI3K inhibitor LY294002 and PARP inhibitor Talazoparib combination in BRCA-deficient triple negative breast cancer cells. Cell Signal. 2022;91: 110229. https://doi.org/10.1016/j.cellsig.2021.110229.
Del Pozo V, Robles AJ, Fontaine SD, Liu Q, Michalek JE, Houghton PJ, Kurmasheva RT. PEGylated talazoparib enhances therapeutic window of its combination with temozolomide in Ewing sarcoma. IScience. 2022;25(2): 103725. https://doi.org/10.1016/j.isci.2021.103725.
DuRoss AN, Landry MR, Thomas CR, Neufeld MJ, Sun C. Fucoidan-coated nanoparticles target radiation-induced P-selectin to enhance chemoradiotherapy in murine colorectal cancer. Cancer Lett. 2021;500:208–19. https://doi.org/10.1016/j.canlet.2020.11.021.
Belz J, Ojo NC, Baldwin P, Kumar R, van de Ven A, Liby K, Cormack R, Makrigiorgos M, Sridhar S. Abstract B30: sustained release of PARP inhibitor talazoparib and chemotherapeutic docetaxel from modified brachytherapy spacers for treatment of breast and prostate cancer. Drug Deliv Nanomed. 2017. https://doi.org/10.1158/1538-7445.EPSO16-B30.
Guney Eskiler G, Cecener G, Egeli U, Tunca B. Synthetically lethal BMN 673 (Talazoparib) loaded solid lipid nanoparticles for BRCA1 mutant triple negative breast cancer. Pharm Res. 2018;35(11):218. https://doi.org/10.1007/s11095-018-2502-6.
Landry MR, DuRoss AN, Neufeld MJ, Hahn L, Sahay G, Luxenhofer R, Sun C. Low dose novel PARP-PI3K inhibition via nanoformulation improves colorectal cancer immunoradiotherapy. Mater Today Bio. 2020;8: 100082. https://doi.org/10.1016/j.mtbio.2020.100082.
Bhattacharjee S, Sullivan MJ, Wynn RR, Demagall A, Hendrix AS, Sindhwani P, Petros FG, Nadiminty N. PARP inhibitors chemopotentiate and synergize with cisplatin to inhibit bladder cancer cell survival and tumor growth. BMC Cancer. 2022;22(1):312. https://doi.org/10.1186/s12885-022-09376-9.
Hu H, Zhang Y, Ji W, Mei H, Wu T, He Z, Wang K, Shi C. Hyaluronic acid-coated and Olaparib-loaded PEI − PLGA nanoparticles for the targeted therapy of triple negative breast cancer. J Microencapsul. 2022;39(1):25–36. https://doi.org/10.1080/02652048.2021.2014586.
Liu Y, Wang M, Liu W, Jing J, Ma H. Olaparib and doxorubicin co-loaded polypeptide nanogel for enhanced breast cancer therapy. Front Bioeng Biotechnol. 2022;10: 904344. https://doi.org/10.3389/fbioe.2022.904344.
Acknowledgements
The authors are grateful to Shoolini university for providing junior research scholarship to Simran Deep Kaur and also to all the researchers who discovered PARP inhibitors and PARP inhibitors drug delivery system that was helpful for framing this review paper.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
Initiation and conceptualization of the study were undertaken by all authors. SDK were responsible for planning and framework of the paper. Preparation of the first draft, all the figures and tables were prepared by SDK. Previous drafts of the article were reviewed and commented by DKC, AAA, MT, KD, DNK. The final version of manuscript is prepared by SDK and DNK. DNK supervised the manuscript. Following several revisions, the final version was unanimously endorsed by all of the authors.
Corresponding author
Ethics declarations
Conflict of interest
Simran Deep Kaur, Dinesh Kumar Chellappan, Alaa A Aljabali, Murtaza Tambuwala, Kamal Dua, Deepak N Kapoor declare that they have no conflict of interest.
Ethical approval
Not applicable.
Consent for publication
We agreed with the journal policy and provided our consent for the publication.
Consent to participate
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaur, S.D., Chellappan, D.K., Aljabali, A.A. et al. Recent advances in cancer therapy using PARP inhibitors. Med Oncol 39, 241 (2022). https://doi.org/10.1007/s12032-022-01840-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-022-01840-7