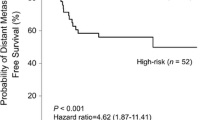
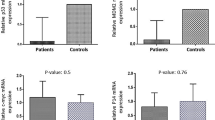
Abstract
Breast cancer is the most common cancer among women in terms of prevalence and mortality, and chemotherapy is one of the most effective treatments at higher stages. However, resistance to chemotherapy is the main obstacle in the treatment of this cancer. Accumulated evidence identified the PD-L1 protein as an essential protein in the development of different cancers. Abnormal expression of this protein in various tumor cells is linked to cancer development and inhibiting the function of immune cells, which correlated with reduced beneficial effects of chemotherapy drugs. In the present study, the effects of common chemotherapy drugs including doxorubicin, paclitaxel, and docetaxel on the expression of the PD-L1 gene were investigated by qRT-PCR before and after the treatment with these drugs in MD231, MD468, SKBR3 breast cancer cell lines. Also, the MTT test was applied to examine the effects of drugs on the growth and proliferation of cancer cells considering PD-L1 expression. The expression of the PD-L1 gene increased after 24 and 48 h of treatment with chemotherapy drugs. The obtained results indicate the enhancing effects of chemotherapy drugs on PD-L1 gene expression, which have a suppressive effect on the immune system against breast cancer. The use of these drugs as the first line of chemotherapy in triple-negative breast cancer is not recommended. However, there is still a need for further experimental and clinical research on the exact effects of these drugs on undesired immune cells exhaustion in breast cancer therapy.






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype. Cancer. 2007;109:1721–8.
Dias K, Dvorkin-Gheva A, Hallett RM, Wu Y, Hassell J, Pond GR, et al. Claudin-low breast cancer; clinical & pathological characteristics. PLoS ONE. 2017;12:e0168669.
Makki J. Diversity of breast carcinoma: histological subtypes and clinical relevance. Clin Med Insights Pathol. 2015. https://doi.org/10.4137/CPath.S31563.
Schnitt SJ. Classification and prognosis of invasive breast cancer: from morphology to molecular taxonomy. Mod Pathol. 2010;23:S60–4.
Derakhshani A, Rezaei Z, Safarpour H, Sabri M, Mir A, Sanati MA, et al. Overcoming trastuzumab resistance in HER2-positive breast cancer using combination therapy. J Cell Physiol. 2020;235:3142–56.
Smid M, Wang Y, Zhang Y, Sieuwerts AM, Yu J, Klijn JGM, et al. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008;68:3108–14.
Dong G, Wang D, Liang X, Gao H, Wang L, Yu X, et al. Factors related to survival rates for breast cancer patients. Int J Clin Exp Med. 2014;7:3719.
Dastmalchi N, Safaralizadeh R, Baradaran B, Hosseinpourfeizi M, Baghbanzadeh A. An update review of deregulated tumor suppressive microRNAs and their contribution in various molecular subtypes of breast cancer. Gene. 2020;729:144301.
Soliman H, Khalil F, Antonia S. PD-L1 expression is increased in a subset of basal type breast cancer cells. PLoS ONE. 2014;9:e88557.
Zheng Y, Fang Y, Li J. PD-L1 expression levels on tumor cells affect their immunosuppressive activity. Oncol Lett. 2019;18(5):5399–407.
Sabatier R, Finetti P, Mamessier E, Adelaide J, Chaffanet M, Ali HR, et al. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget. 2015;6:5449–64.
Howard JH, Bland KI. Current management and treatment strategies for breast cancer. Curr Opin Obstet Gynecol. 2012;24:44–8.
Hassan MSU. Chemotherapy for breast cancer (review). Oncol Rep. 2010;24:1121–31.
Martin AM, Nirschl TR, Nirschl CJ, Francica BJ, Kochel CM, van Bokhoven A, et al. Paucity of PD-L1 expression in prostate cancer: innate and adaptive immune resistance. Prostate Cancer Prostatic Dis. 2015;18:325–32.
Ng HY, Li J, Tao L, Lam AK-Y, Chan KW, Ko JMY, et al. Chemotherapeutic treatments increase PD-L1 expression in esophageal squamous cell carcinoma through EGFR/ERK activation. Transl Oncol. 2018;11:1323–33.
Ghebeh H, Mohammed S, Al-Omair A, Qattant A, Lehe C, Al-Qudaihi G, et al. The B7–H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006;8:190–8.
Lotfinejad P, Kazemi T, Safaei S, Amini M, Roshani asl E, Baghbani E, et al. PD-L1 silencing inhibits triple-negative breast cancer development and upregulates T-cell-induced pro-inflammatory cytokines. Biomed Pharmacother. 2021;138:111436.
Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2:361–70.
Bailly C, Thuru X, Quesnel B. Combined cytotoxic chemotherapy and immunotherapy of cancer: modern times. NAR Cancer. 2020;2:zcaa002.
Yang M, Liu P, Wang K, Glorieux C, Hu Y, Wen S, et al. Chemotherapy induces tumor immune evasion by upregulation of programmed cell death ligand 1 expression in bone marrow stromal cells. Mol Oncol. 2017;11:358–72.
Zhang P, Su D-M, Liang M, Fu J. Chemopreventive agents induce programmed death-1-ligand 1 (PD-L1) surface expression in breast cancer cells and promote PD-L1-mediated T cell apoptosis. Mol Immunol. 2008;45:1470–6.
Funaki S, Shintani Y, Kawamura T, Kanzaki R, Minami M, Okumura M. Chemotherapy enhances programmed cell death 1/ligand 1 expression via TGF-β induced epithelial mesenchymal transition in non-small cell lung cancer. Oncol Rep. 2017;38:2277–84.
Gu Z, Wang Q, Shi Y, Huang Y, Zhang J, Zhang X, et al. Nanotechnology-mediated immunochemotherapy combined with docetaxel and PD-L1 antibody increase therapeutic effects and decrease systemic toxicity. J Control Release. 2018;286:369–80.
Nounou MI, ElAmrawy F, Ahmed N, Abdelraouf K, Goda S, Syed-Sha-Qhattal H. Breast cancer: conventional diagnosis and treatment modalities and recent patents and technologies. Breast Cancer Basic Clin Res. 2015;9(2):17–34.
Lotfinejad P, Kazemi T, Mokhtarzadeh A, Shanehbandi D, Jadidi Niaragh F, Safaei S, et al. PD-1/PD-L1 axis importance and tumor microenvironment immune cells. Life Sci. 2020;259:118297.
Acknowledgements
We thank the research staff at the Immunology Research Center, Tabriz University of Medical Sciences for assistance in performing experiments.
Funding
This work was financially supported by a grant from Tabriz University of Medical Sciences, Tabriz, Iran (Project No. 62280).
Author information
Authors and Affiliations
Contributions
MM, and BB devised the main conceptual ideas and participated in the design of the work. BB provided biological materials and reagents. MM, SS, MA, and AB performed the experiments. MM, and KH wrote the initial draft of the manuscript. SH, SSS, and AM participated in the analysis of the work and reviewed and edited the manuscript. BB supervised the study.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
All experiments and procedures were conducted in compliance with the ethical principles of Tabriz University of Medical Science, Tabriz, Iran and approved by the regional ethical committee for medical research.
Consent to participate
This article does not contain any studies with human or animal subjects performed by any of the authors.
Consent for publication
All authors agree with publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Majidi, M., Safaee, S., Amini, M. et al. The effects of chemotherapeutic drugs on PD-L1 gene expression in breast cancer cell lines. Med Oncol 38, 147 (2021). https://doi.org/10.1007/s12032-021-01556-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-021-01556-0