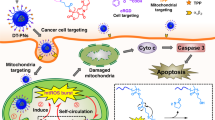
Abstract
Cancer is one of the diseases that threatens human health and is a leading cause of mortality worldwide. High levels of reactive oxygen species (ROS) have been observed in cancer tissues compared with normal tissues in vivo, and it is not yet known how this influences chemotherapeutic drug action. Cationic porphyrin 5,10,15,20-tetra-(N-methyl-4-pyridyl) porphyrin (TMPyP4) is a photosensitizer used in photodynamic therapy (PDT) and a telomerase inhibitor used in the treatment of telomerase-positive cancer. Here, we investigated the anticancer activity of TMPyP4 in A549 and PANC cells cultured in H2O2. The results showed that compared to TMPyP4 alone, the combination of TMPyP4 and H2O2 exhibited sensitization effects on cell viability and colony formation inhibition and apoptosis in A549 and PANC cells, but had no effect in human normal MIHA cells. Mechanistically, the combination of TMPyP4 and H2O2 activates high ROS and mitochondrial membrane potential in A549 and PANC cells, resulting in intense DNA damage and DNA damage responses. Consequently, compared to TMPyP4 alone, TMPyP4 and H2O2 combined treatment upregulates the expression of BAX, cleaved caspase 3, and p-JNK and downregulates the expression of Bcl-2 in A549 and PANC cells. Taken together, these data suggested that H2O2 enhanced the anticancer activity of TMPyP4-mediated ROS-dependent DNA damage and related apoptotic protein regulation, revealing that the high ROS tumor microenvironment plays an important role in chemotherapeutic drug action.






Similar content being viewed by others
Data availability
The data are real reliable.
Abbreviations
- TME:
-
Tumor microenvironment
- ROS:
-
Reactive oxygen species
- PDT:
-
Photodynamic therapy
- TMPyP4:
-
5,10,15,20-Tetra-(N-methyl-4-pyridyl) porphyrin
- PDT:
-
Photodynamic therapy
- MTT:
-
3‑(4,5‑Dimethyl‑2‑thiazolyl)‑2,5‑diphenyl‑2‑H‑tetrazolium bromide
- FCM:
-
Flow cytometry
- IF:
-
Immunofluorescence
References
Weinberg F, Ramnath N, Nagrath D. Reactive oxygen species in the tumor microenvironment: an overview. Cancers. 2019. https://doi.org/10.3390/cancers11081191.
Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51(3):794–8.
Lopez-Lazaro M. Dual role of hydrogen peroxide in cancer: possible relevance to cancer chemoprevention and therapy. Cancer Lett. 2007;252(1):1–8. https://doi.org/10.1016/j.canlet.2006.10.029.
Buettner GR, Ng CF, Wang M, Rodgers VG, Schafer FQ. A new paradigm: manganese superoxide dismutase influences the production of H2O2 in cells and thereby their biological state. Free Radic Biol Med. 2006;41(8):1338–50. https://doi.org/10.1016/j.freeradbiomed.2006.07.015.
Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci USA. 2010;107(19):8788–93. https://doi.org/10.1073/pnas.1003428107.
Weinberg F, Chandel NS. Reactive oxygen species-dependent signaling regulates cancer. Cell Mol Life Sci. 2009;66(23):3663–73. https://doi.org/10.1007/s00018-009-0099-y.
Okon IS, Coughlan KA, Zhang M, Wang Q, Zou MH. Gefitinib-mediated reactive oxygen specie (ROS) instigates mitochondrial dysfunction and drug resistance in lung cancer cells. J Biol Chem. 2015;290(14):9101–10. https://doi.org/10.1074/jbc.M114.631580.
Dharmaraja AT. Role of reactive oxygen species (ROS) in therapeutics and drug resistance in cancer and bacteria. J Med Chem. 2017;60(8):3221–40. https://doi.org/10.1021/acs.jmedchem.6b01243.
Cui Q, Wang JQ, Assaraf YG, Ren L, Gupta P, Wei L, et al. Modulating ROS to overcome multidrug resistance in cancer. Drug Resist Updat. 2018;41:1–25. https://doi.org/10.1016/j.drup.2018.11.001.
Yang H, Villani RM, Wang H, Simpson MJ, Roberts MS, Tang M, et al. The role of cellular reactive oxygen species in cancer chemotherapy. J Exp Clin Cancer Res. 2018;37(1):266. https://doi.org/10.1186/s13046-018-0909-x.
Pervaiz S, Olivo M. Art and science of photodynamic therapy. Clin Exp Pharmacol Physiol. 2006;33(5–6):551–6. https://doi.org/10.1111/j.1440-1681.2006.04406.x.
Benov L. Photodynamic therapy: current status and future directions. Med Princ Pract. 2015;24(Suppl 1):14–28. https://doi.org/10.1159/000362416.
Debele TA, Peng S, Tsai HC. Drug carrier for photodynamic cancer therapy. Int J Mol Sci. 2015;16(9):22094–136. https://doi.org/10.3390/ijms160922094.
Pushpan SK, Venkatraman S, Anand VG, Sankar J, Parmeswaran D, Ganesan S, et al. Porphyrins in photodynamic therapy: a search for ideal photosensitizers. Curr Med Chem Anticancer Agents. 2002;2(2):187–207. https://doi.org/10.2174/1568011023354137.
Villanueva A, Caggiari L, Jori G, Milanesi C. Morphological aspects of an experimental tumour photosensitized with a meso-substituted cationic porphyrin. J Photochem Photobiol B. 1994;23(1):49–56. https://doi.org/10.1016/1011-1344(93)06982-9.
Awan MA, Tarin SA. Review of photodynamic therapy. Surgeon. 2006;4(4):231–6. https://doi.org/10.1016/s1479-666x(06)80065-x.
Tada-Oikawa S, Oikawa S, Hirayama J, Hirakawa K, Kawanishi S. DNA damage and apoptosis induced by photosensitization of 5,10,15,20-tetrakis (N-methyl-4-pyridyl)-21H,23H-porphyrin via singlet oxygen generation. Photochem Photobiol. 2009;85(6):1391–9. https://doi.org/10.1111/j.1751-1097.2009.00600.x.
Konieczna N, Romaniuk-Drapala A, Lisiak N, Toton E, Paszel-Jaworska A, Kaczmarek M, et al. Telomerase inhibitor TMPyP4 alters adhesion and migration of breast-cancer cells MCF7 and MDA-MB-231. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20112670.
Zheng XH, Nie X, Liu HY, Fang YM, Zhao Y, Xia LX. TMPyP4 promotes cancer cell migration at low doses, but induces cell death at high doses. Sci Rep. 2016;6:26592. https://doi.org/10.1038/srep26592.
Mikami-Terao Y, Akiyama M, Yuza Y, Yanagisawa T, Yamada O, Yamada H. Antitumor activity of G-quadruplex-interactive agent TMPyP4 in K562 leukemic cells. Cancer Lett. 2008;261(2):226–34. https://doi.org/10.1016/j.canlet.2007.11.017.
Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70(2):440–6. https://doi.org/10.1158/0008-5472.Can-09-1947.
Zheng XH, Nie X, Fang YM, Zhang ZP, Xiao YN, Mao ZW, et al. A cisplatin derivative tetra-Pt(bpy) as an oncotherapeutic agent for targeting ALT cancer. J Natl Cancer I. 2017. https://doi.org/10.1093/jnci/djx061.
Olive PL, Banath JP. The comet assay: a method to measure DNA damage in individual cells. Nat Protoc. 2006;1(1):23–9. https://doi.org/10.1038/nprot.2006.5.
Park WH. MAPK inhibitors, particularly the JNK inhibitor, increase cell death effects in H2O2-treated lung cancer cells via increased superoxide anion and glutathione depletion. Oncol Rep. 2018;39(2):860–70. https://doi.org/10.3892/or.2017.6107.
Rhee SG, Kang SW, Jeong W, Chang TS, Yang KS, Woo HA. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol. 2005;17(2):183–9. https://doi.org/10.1016/j.ceb.2005.02.004.
Sies H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol. 2017;11:613–9. https://doi.org/10.1016/j.redox.2016.12.035.
Wang WJ, Mao LF, Lai HL, Wang YW, Jiang ZB, Li W, et al. Dolutegravir derivative inhibits proliferation and induces apoptosis of non-small cell lung cancer cells via calcium signaling pathway. Pharmacol Res. 2020;161:105129. https://doi.org/10.1016/j.phrs.2020.105129.
Weinberg SE, Chandel NS. Targeting mitochondria metabolism for cancer therapy. Nat Chem Biol. 2015;11(1):9–15. https://doi.org/10.1038/nchembio.1712.
Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci USA. 2003;100(5):2432–7. https://doi.org/10.1073/pnas.0438011100.
Ma L, Wei J, Wan J, Wang W, Wang L, Yuan Y, et al. Low glucose and metformin-induced apoptosis of human ovarian cancer cells is connected to ASK1 via mitochondrial and endoplasmic reticulum stress-associated pathways. J Exp Clin Cancer Res. 2019. https://doi.org/10.1186/s13046-019-1090-6.
Chen X, Zhao Y, Luo W, Chen SA, Lin F, Zhang X, et al. Celastrol induces ROS-mediated apoptosis via directly targeting peroxiredoxin-2 in gastric cancer cells. Theranostics. 2020;10(22):10290–308. https://doi.org/10.7150/thno.46728.
Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8(7):579–91. https://doi.org/10.1038/nrd2803.
Srinivas US, Tan BWQ, Vellayappan BA, Jeyasekharan AD. ROS and the DNA damage response in cancer. Redox Biol. 2019;25:101084. https://doi.org/10.1016/j.redox.2018.101084.
Sies H. Role of reactive oxygen species in biological processes. Klin Wochenschr. 1991;69(21–23):965–8. https://doi.org/10.1007/BF01645140.
Ly JD, Grubb DR, Lawen A. The mitochondrial membrane potential (deltapsi(m)) in apoptosis; an update. Apoptosis. 2003;8(2):115–28. https://doi.org/10.1023/a:1022945107762.
Shim HY, Park JH, Paik HD, Nah SY, Kim DS, Han YS. Acacetin-induced apoptosis of human breast cancer MCF-7 cells involves caspase cascade, mitochondria-mediated death signaling and SAPK/JNK1/2-c-Jun activation. Mol Cells. 2007;24(1):95–104.
Liu J, Lin A. Role of JNK activation in apoptosis: a double-edged sword. Cell Res. 2005;15(1):36–42. https://doi.org/10.1038/sj.cr.7290262.
Acknowledgements
We thank Elsevier (http://webshop.elsevier.com/languageediting/) for its linguistic assistance during the preparation of this manuscript.
Funding
The present study was funded by National Science Foundation of China (Grant No.: 21701194), Wenzhou Basic Medical and Health Technology Project (Grant No.: Y20180177), and Wenzhou Medical University Talent Start-up Fund (Grant No.: QTJ17022) for the financial support.
Author information
Authors and Affiliations
Contributions
Conceptualization: XHZ, GL, XDZ, and JQC. Methodology and Data analysis: JQC, XXJ, ZS, YM, and JFZ. Writing—original draft preparation: XZ and JC. Funding acquisition and supervision: XHZ, GL, and XDZ.
Corresponding authors
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Informed consent
All authors have approved this work. All authors agree to publish.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, J., Jin, X., Shen, Z. et al. H2O2 enhances the anticancer activity of TMPyP4 by ROS-mediated mitochondrial dysfunction and DNA damage. Med Oncol 38, 59 (2021). https://doi.org/10.1007/s12032-021-01505-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-021-01505-x