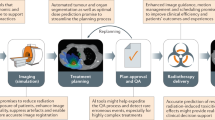
Abstract
Recent advances in computing capability allowed the development of sophisticated predictive models to assess complex relationships within observational data, described as Artificial Intelligence. Medicine is one of the several fields of application and Radiation oncology could benefit from these approaches, particularly in patients’ medical records, imaging, baseline pathology, planning or instrumental data. Artificial Intelligence systems could simplify many steps of the complex workflow of radiotherapy such as segmentation, planning or delivery. However, Artificial Intelligence could be considered as a “black box” in which human operator may only understand input and output predictions and its application to the clinical practice remains a challenge. The low transparency of the overall system is questionable from manifold points of view (ethical included). Given the complexity of this issue, we collected the basic definitions to help the clinician to understand current literature, and overviewed experiences regarding implementation of AI within radiotherapy clinical workflow, aiming to describe this field from the clinician perspective.

Similar content being viewed by others
References
El Naqa I, et al. Artificial Intelligence: reshaping the practice of radiological sciences in the 21st century. Br J Radiol. 2020;93:1106.
Meyer P, et al. Survey on deep learning for radiotherapy. Comput Biol Med. 2018;98:126–46.
Sharp G, et al. Vision 20/20: perspectives on automated image segmentation for radiotherapy. Med Phys. 2014;5:050902.
Eldesoky AR, et al. Internal and external validation of an ESTRO delineation guideline: dependent automated segmentation tool for loco-regional radiation therapy of early breast cancer. Radiother Oncol. 2016;3:424–30.
Nguyen A et al. Deep neural networks are easily fooled: high confidence predictions for unrecognizable images. In: Proc. IEEE Conf. Comput. Vis. Pattern Recognit; 2015; pp. 427–436.
Charron O, et al. Automatic detection and segmentation of brain metastases on multimodal MR images with a deep convolutional neural network. Comput Biol Med. 2018;95:43–544.
Shickel B, et al. Deep EHR: a survey of recent advances in deep learning techniques for electronic health record (EHR) analysis. IEEE J Biomed Health Inform. 2018;22(5):1589–604.
Boden MA. Artificial intelligence and natural man. New York: Basic Books; 1977.
Tsay D, Patterson C. From machine learning to artificial intelligence applications in cardiac care. Circulation. 2018;138:2569–75.
LeCun Y, et al. Deep learning. Nature. 2015;521:436–44.
Mitchell TM. Machine learning. New York: McGraw-Hill; 1997.
Speight, et al. Evaluation of atlas based auto-segmentation for head and neck target volume delineation in adaptive/replan IMRT. J Phys Conf Ser. 2014;489:012060.
Men K, et al. Deep deconvolutional neural network for target segmentation of nasopharyngeal cancer in planning computed tomography images. Front Oncol. 2017;7:315.
Cardenas CE, et al. Deep learning algorithm for auto-delineation of high-risk oropharyngeal clinical target volumes with built-in dice similarity coefficient parameter optimization function. Int J Radiat Oncol Biol Phys. 2018;101:468–78.
McCarroll RE, et al. Retrospective validation and clinical implementation of automated contouring of organs at risk in the head and neck: a step toward automated radiation treatment planning for low- and middle-income countries. J Glob Oncol. 2018;4:1–11.
Nikolov S et al. Deep learning to achieve clinically applicable segmentation of head and neck anatomy for radiotherapy. ArXiv, 2018; arXiv:1809.04430.
Li Q, et al. Tumor segmentation in contrast-enhanced magnetic resonance imaging for nasopharyngeal carcinoma: deep learning with convolutional neural network. Biomed Res Int. 2018. https://doi.org/10.1155/2018/9128527.
Tong N, et al. Fully automatic multi-organ segmentation for head and neck cancer radiotherapy using shape representation model constrained fully convolutional neural networks. Med Phy. 2018;45(10):4558–677.
Van Rooij W, et al. Deep learning-based delineation of head and neck organs at risk: geometric and dosimetric evaluation. Int J Radiat Oncol Biol Phys. 2019;104(3):677–84.
Van Dijk LV, et al. Improving automatic delineation for head and neck organs at risk by Deep Learning Contouring. Radiother Oncol. 2020;142:115–23.
Martin S, et al. A multiphase validation of atlas-based automatic and semiautomatic segmentation strategies for prostate MRI. Int J Radiat Oncol Biol Phys. 2013;85:95–100.
Macomber MW, et al. Autosegmentation of prostate anatomy for radiation treatment planning using deep decision forests of radiomic features. Phys Med Biol. 2018;63(23):235002.
Lustberg T, et al. Clinical evaluation of atlas and deep learning based automatic contouring for lung cancer. Radiother Oncol. 2018;126:312–7.
Men K, et al. Automatic segmentation of the clinical target volume and organs at risk in the planning CT for rectal cancer using deep dilated convolutional neural networks. Med Phys. 2017;44(12):6377–89.
Liu Y, et al. A deep convolutional neural network-based automatic delineation strategy for multiple brain metastases stereotactic radiosurgery. PLoS ONE. 2017;12(10):e0185844.
Men K, et al. Fully automatic and robust segmentation of the clinical target volume for radiotherapy of breast cancer using big data and deep learning. Phys Med. 2018;50:13–9.
Menze BH, et al. The multimodal brain tumor image segmentation benchmark (BRATS). IEEE Trans Med Imaging. 2015;34:1993–2024.
Fan J, et al. Automatic treatment planning based on three-dimensional dose distribution predicted from deep learning technique. Med Phys. 2019;1:370–81.
Chen X, et al. A feasibility study on an automated method to generate patient-specific dose distributions for radiotherapy using deep learning. Med Phys. 2019;1:56–64.
Liu F, et al. MR-based treatment planning in radiation therapy using a deep learning approach. J Appl Clin Med Phys. 2019;20(3):105–14.
Ma M, et al. Dosimetric features-driven machine learning model for DVH prediction in VMAT treatment planning. Med Phys. 2019;2:857–67.
Barragán-Montero AM, et al. Three-dimensional dose prediction for lung IMRT patients with deep neural networks: robust learning from heterogeneous beam configurations. Med Phys. 2019;46(8):3679–91.
Bai X, et al. Approach and assessment of automated stereotactic radiotherapy planning for early stage non-small-cell lung cancer. Biomed Eng Online. 2019;18(1):101.
Carlson JN, et al. A machine learning approach to the accurate prediction of multi-leaf collimator positional errors. Phys Med Biol. 2016;61(6):2514–31.
Liu Z, et al. A deep learning method for prediction of three-dimensional dose distribution of helical tomotherapy. Med Phys. 2019;46(5):1972–83.
Osman AFI, et al. Prediction of the individual multileaf collimator positional deviations during dynamic IMRT delivery priori with artificial neural network. Med Phys. 2020. https://doi.org/10.1002/mp.14014.
Mahdavi SR, et al. Use of artificial neural network for pretreatment verification of intensity modulation radiation therapy fields. Br J Radiol. 2019;92(1102):20190355. https://doi.org/10.1259/bjr.20190355.
Zhao W, et al. Markerless pancreatic tumor target localization enabled by deep learning. Int J Radiat Oncol Biol Phys. 2019;105(2):432–9.
Malone C, et al. Using a neural network to predict deviations in mean heart dose during the treatment of left-sided deep inspiration breath hold patients. Phys Med. 2019;65:137–42.
Kann BH, Aneja S, Loganadane GV, Kelly JR, Smith SM, Decker RH, et al. Pretreatment identification of head and neck cancer nodal metastasis and extranodal extension using deep learning neural networks. Sci Rep. 2018;8:14036. https://doi.org/10.1038/s41598-018-32441-y.
Li S, Wang K, Hou Z, Yang J, Ren W, Gao S, Meng F, Wu P, Liu B, Liu J, Yan J. Use of radiomics combined with machine learning method in the recurrence patterns after intensity-modulated radiotherapy for nasopharyngeal carcinoma: a preliminary study. Front Oncol. 2018;8:648. https://doi.org/10.3389/fonc.2018.00648.
Baek S, He Y, Allen BG, Buatti JM, Smith BJ, Tong L, Sun Z, Wu J, Diehn M, Loo BW, Plichta KA, Seyedin SN, Gannon M, Cabel KR, Kim Y, Wu X. Deep segmentation networks predict survival of non-small cell lung cancer. Sci Rep. 2019;9(1):17286. https://doi.org/10.1038/s41598-019-53461-2.
Bibault JE, Giraud P, Housset M, Durdux C, Taieb J, Berger A, Coriat R, Chaussade S, Dousset B, Nordlinger B, Burgun A. Deep learning and radiomics predict complete response after neo-adjuvant chemoradiation for locally advanced rectal cancer. Sci Rep. 2018;8(1):12611.
Gabryś HS, et al. Design and selection of machine learning methods using radiomics and dosiomics for normal tissue complication probability modeling of xerostomia. Front Oncol. 2018;8:35.
Jiang W, et al. Machine learning methods uncover radiomorphologic dose patterns in salivary glands that predict xerostomia in patients with head and neck cancer. Adv Radiat Oncol. 2018;4:401–12.
Men K, et al. A deep learning model for predicting xerostomia due to radiation therapy for head and neck squamous cell carcinoma in the RTOG 0522 clinical trial. Int J Radiat Oncol Biol Phys. 2019;2:440–7.
Lee S, et al. Machine learning on a genome-wide association study to predict late genitourinary toxicity after prostate radiation therapy. Int J Radiat Oncol Biol Phys. 2018;101:128–35.
Tian Z, et al. A machine-learning-based prediction model of fistula formation after interstitial brachytherapy for locally advanced gynecological malignancies. Brachytherapy. 2019;4:530–8.
Tseng HH, Luo Y, Cui S, Chien JT, Ten Haken RK, Naqa IE. Deep reinforcement learning for automated radiation adaptation in lung cancer. Med Phys. 2017;44(12):6690–705.
Vayena E, Blasimme A, Cohen IG. Machine learning in medicine: Addressing ethical challenges. PLoS Med. 2018;15(11):e1002689. https://doi.org/10.1371/journal.pmed.1002689.
Price WN. Regulating black-box medicine. Mich L Rev. 2017;116(1):421–74.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Francolini, G., Desideri, I., Stocchi, G. et al. Artificial Intelligence in radiotherapy: state of the art and future directions. Med Oncol 37, 50 (2020). https://doi.org/10.1007/s12032-020-01374-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-020-01374-w