Abstract
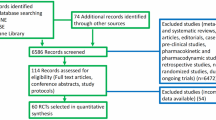
Immune checkpoint inhibitors (CKIs) are therapeutic weapons in several advanced malignancies. Performance status is a validated prognostic variable in cancer patients; it possibly affects the efficiency of the immune system. We performed a systematic review and meta-analysis to investigate the predictive role of PS toward treatment with CKIs in cancer patients. Following PRISMA guidelines, an electronic search from PubMed, The Cochrane Library and Embase was performed, from the inception of each database to May 31, 2018. Inclusion criteria were (1) randomized trials comparing CKI with standard therapy for the treatment of patients with solid tumors; (2) information on overall survival (OS) according to PS; (3) full text available; and (4) reported in English language. Data were pooled using HRs for OS according to random effect model. The effect of experimental versus control arms was evaluated in PS = 0 and 1–2 subgroups, and the heterogeneity between the two estimates was assessed using an interaction test. The OS differences between PS = 0 and PS = 1–2 strata were evaluated in all studies and according to predefined subgroups. Eighteen studies were eligible, with 11,354 patients [PS = 0 group 5217 patients (46%); PS = 1–2 group 6137 patients (54%)]. The pooled HR for OS was 0.78 (95% CI 0.69–0.89) in PS = 0 patients. In PS = 1–2 patients, the pooled OS HR was 0.78 (95% CI 0.71–0.86). The OS difference between PS = 0 and PS = 1–2 patients treated with CKI was not significant (P = 0.99). CKI improves survival irrespective of patients’ PS. PS should not guide treatment choice for anticancer immunotherapy.



Similar content being viewed by others
References
Bersanelli M, Buti S. From targeting the tumor to targeting the immune system: transversal challenges in oncology with the inhibition of the PD-1/PD-L1 axis. World J Clin Oncol. 2017;8(1):37–53. https://doi.org/10.5306/wjco.v8.i1.37.
Elias R, Giobbie-Hurder A, McCleary NJ, Ott P, Hodi FS, Rahma O. Efficacy of PD-1 & PD-L1 inhibitors in older adults: a meta-analysis. J Immunother Cancer. 2018;6(1):26. https://doi.org/10.1186/s40425-018-0336-8.
Conforti F, Pala L, Bagnardi V, De Pas T, Martinetti M, Viale G, Gelber RD, Goldhirsch A. Cancer immunotherapy efficacy and patients’ sex: a systematic review and meta-analysis. Lancet Oncol. 2018. https://doi.org/10.1016/S1470-2045(18)30261-4.
Cortellini A, Bersanelli M, Buti S, Gambale E, Atzori F, Zoratto F, Parisi A, Brocco D, Pireddu A, Cannita K, Iacono D, Migliorino MR, Gamucci T, De Tursi M, Sidoni T, Tiseo M, Michiara M, Papa A, Angius G, Tomao S, Fargnoli MC, Natoli C, Ficorella C. Family history of cancer as surrogate predictor for immunotherapy with anti-PD1/PD-L1 agents: preliminary report of the FAMI-L1 study. Immunotherapy. 2018 https://doi.org/10.2217/imt-2017-0167.
Kim JH, Kim HS, Kim BJ. Prognostic value of smoking status in non-small-cell lung cancer patients treated with ICI: a meta-analysis. Oncotarget. 2017;8(54):93149–55. https://doi.org/10.18632/oncotarget.18703.
Elenkov IJ, Chrousos GP. Stress system-organization, physiology and immunoregulation. Neuroimmunomodulation. 2006;13(5–6):257–67.
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55.
Jang RW, Caraiscos VB, Swami N, Banerjee S, Mak E, Kaya E, Rodin G, Bryson J, Ridley JZ, Le LW, Zimmermann C. Simple prognostic model for patients with advanced cancer based on performance status. J Oncol Pract. 2014;10(5):e335-41. https://doi.org/10.1200/JOP.2014.001457.
Wang L, Shen Y. Imbalance of circulating T-lymphocyte subpopulation in gastric cancer patients correlated with performance status. Clin Lab. 2013;59(3–4):429–33.
Campbell J, Turner J. Debunking the myth of exercise-induced immune suppression: redefining the impact of exercise on immunological health across the lifespan. Front Immunol. 2018, https://doi.org/10.3389/fimmu.2018.00648.
Abdalla DR, Murta EF, Michelin MA. The influence of physical activity on the profile of immune response cells and cytokine synthesis in mice with experimental breast tumors induced by 7,12-dimethylbenzanthracene. Eur J Cancer Prev. 2013;22(3):251–8. https://doi.org/10.1097/CEJ.0b013e3283592cbb.
Okuma Y, Hosomi Y, Nagamata M, Yamada Y, Sekihara K, Kato K, Hishima T, Okamura T. Clinical outcomes after first-line EGFR inhibitor treatment for patients with NSCLC, EGFR mutation, and poor performance status. Anticancer Res. 2013;33(11):5057–64.
Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. https://doi.org/10.1016/0197-2456(95)00134-4.
Begg CB, Mazumdar M. Operating characteristics of a bank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. https://doi.org/10.2307/2533446.
Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, Davidson N, Richards J, Maio M, Hauschild A, Miller WH Jr, Gascon P, Lotem M, Harmankaya K, Ibrahim R, Francis S, Chen TT, Humphrey R, Hoos A, Wolchok JD. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–26. https://doi.org/10.1056/NEJMoa1104621.
Ribas A, Kefford R, Marshall MA, Punt CJ, Haanen JB, Marmol M, Garbe C, Gogas H, Schachter J, Linette G, Lorigan P, Kendra KL, Maio M, Trefzer U, Smylie M, McArthur GA, Dreno B, Nathan PD, Mackiewicz J, Kirkwood JM, Gomez-Navarro J, Huang B, Pavlov D, Hauschild A. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31(5):616–22. https://doi.org/10.1200/JCO.2012.44.6112.
Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, Krainer M, Houede N, Santos R, Mahammedi H, Ng S, Maio M, Franke FA, Sundar S, Agarwal N, Bergman AM, Ciuleanu TE, Korbenfeld E, Sengeløv L, Hansen S, Logothetis C, Beer TM, McHenry MB, Gagnier P, Liu D, Gerritsen WR. CA184-043 Investigators. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15(7):700–12. https://doi.org/10.1016/S1470-2045(14)70189-5.
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crinò L, Blumenschein GR Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–39. https://doi.org/10.1056/NEJMoa1507643.
Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, Savage KJ, Hernberg MM, Lebbé C, Charles J, Mihalcioiu C, Chiarion-Sileni V, Mauch C, Cognetti F, Arance A, Schmidt H, Schadendorf D, Gogas H, Lundgren-Eriksson L, Horak C, Sharkey B, Waxman IM, Atkinson V, Ascierto PA. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–30. https://doi.org/10.1056/NEJMoa1412082.
Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Arén Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–35. https://doi.org/10.1056/NEJMoa1504627.
Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Iglesias Docampo LC, Haddad R, Rordorf T, Kiyota N, Tahara M, Monga M, Lynch M, Geese WJ, Kopit J, Shaw JW, Gillison ML. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–67.
Reck M, Luft A, Szczesna A, Havel L, Kim SW, Akerley W, Pietanza MC, Wu YL, Zielinski C, Thomas M, Felip E, Gold K, Horn L, Aerts J, Nakagawa K, Lorigan P, Pieters A, Kong Sanchez T, Fairchild J, Spigel D. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol. 2016;34(31):3740–8. https://doi.org/10.1200/JCO.2016.67.6601.
Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G Jr, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–50. https://doi.org/10.1016/S0140-6736(15)01281-7.
Govindan R, Szczesna A, Ahn MJ, Schneider CP, Gonzalez Mella PF, Barlesi F, Han B, Ganea DE, Von Pawel J, Vladimirov V, Fadeeva N, Lee KH, Kurata T, Zhang L, Tamura T, Postmus PE, Jassem J, O’Byrne K, Kopit J, Li M, Tschaika M, Reck M. Phase III trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non-small-cell lung cancer. J Clin Oncol. 2017;35(30):3449–57. https://doi.org/10.1200/JCO.2016.71.7629.
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Chen LT. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461–71. https://doi.org/10.1016/S0140-6736(17)31827-5.
Hamid O, Puzanov I, Dummer R, Schachter J, Daud A, Schadendorf D, Blank C, Cranmer LD, Robert C, Pavlick AC, Gonzalez R, Hodi FS, Ascierto PA, Salama AKS, Margolin KA, Gangadhar TC, Wei Z, Ebbinghaus S, Ibrahim N, Ribas A. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur J Cancer. 2017;86:37–45. https://doi.org/10.1016/j.ejca.2017.07.022.
Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, Necchi A, Gerritsen W, Gurney H, Quinn DI, Culine S, Sternberg CN, Mai Y, Poehlein CH, Perini RF, Bajorin DF. KEYNOTE-045 investigators. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;16(11):1015–26. 376(.
Beer TM, Kwon ED, Drake CG, Fizazi K, Logothetis C, Gravis G, Ganju V, Polikoff J, Saad F, Humanski P, Piulats JM, Gonzalez Mella P, Ng SS, Jaeger D, Parnis FX, Franke FA, Puente J, Carvajal R, Sengeløv L, McHenry MB, Varma A, van den Eertwegh AJ, Gerritsen W. J Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. Clin Oncol. 2017;35(1):40–7. Epub 2016 Oct 31.
Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, Felip E, van den Heuvel MM, Ciuleanu TE, Badin F, Ready N, Hiltermann TJN, Nair S, Juergens R, Peters S, Minenza E, Wrangle JM, Rodriguez-Abreu D, Borghaei H, Blumenschein GR Jr, Villaruz LC, Havel L, Krejci J, Corral Jaime J, Chang H, Geese WJ, Bhagavatheeswaran P, Chen AC, Socinski MA, CheckMate 026 Investigators. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415–26. https://doi.org/10.1056/NEJMoa1613493.
Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, Cortinovis DL, Leach J, Polikoff J, Barrios C, Kabbinavar F, Frontera OA, De Marinis F, Turna H, Lee JS, Ballinger M, Kowanetz M, He P, Chen DS, Sandler A, Gandara DR, OAK Study Group. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–65. https://doi.org/10.1016/S0140-6736(16)32517-X.
Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, Cheng SY, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Hui R, Garon EB, Boyer M, Rubio-Viqueira B, Novello S, Kurata T, Gray JE, Vida J, Wei Z, Yang J, Raftopoulos H, Pietanza MC, Garassino MC, KEYNOTE-189 Investigators. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–92. https://doi.org/10.1056/NEJMoa1801005.
Powles T, Durán I, van der Heijden MS, Loriot Y, Vogelzang NJ, De Giorgi U, Oudard S, Retz MM, Castellano D, Bamias A, Fléchon A, Gravis G, Hussain S, Takano T, Leng N, Kadel EE 3rd, Banchereau R, Hegde PS, Mariathasan S, Cui N, Shen X, Derleth CL, Green MC, Ravaud A. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018;391(10122):748–57. https://doi.org/10.1016/S0140-6736(17)33297-X.
Caires-Lima R, Cayres K, Protásio B, Caires I, Andrade J, Rocha L, Takahashi TK, Hoff PM, de Castro G Jr, Mak MP. Palliative chemotherapy outcomes in patients with ECOG-PS higher than 1. Ecancermedicalscience. 2018;12:831. https://doi.org/10.3332/ecancer.2018.831.
Strøm HH, Bremnes RM, Sundstrøm SH, Helbekkmo N, Aasebø U. Poor prognosis patients with inoperable locally advanced NSCLC and large tumors benefit from palliative chemoradiotherapy: a subset analysis from a randomized clinical phase III trial. J Thorac Oncol. 2014;9(6):825–33. https://doi.org/10.1097/JTO.0000000000000184.
Morabito A, Gebbia V, Di Maio M, Cinieri S, Viganò MG, Bianco R, Barbera S, Cavanna L, De Marinis F, Montesarchio V, Costanzo R, Sandomenico C, Montanino A, Mancuso G, Russo P, Nacci A, Giordano P, Daniele G, Piccirillo MC, Rocco G, Gridelli C, Gallo C, Perrone F. Randomized phase III trial of gemcitabine and cisplatin vs. gemcitabine alone in patients with advanced non-small cell lung cancer and a performance status of 2: the CAPPA-2 study. Lung Cancer. 2013;81(1):77–83. https://doi.org/10.1016/j.lungcan.2013.04.008.
Mörth C, Valachis A. Single-agent versus combination chemotherapy as first-line treatment for patients with advanced non-small cell lung cancer and performance status 2: a literature-based meta-analysis of randomized studies. Lung Cancer. 2014;84(3):209–14. https://doi.org/10.1016/j.lungcan.2014.03.015.
Tan PS, Lopes G, Acharyya S, Bilger M, Haaland B. Bayesian network meta-comparison of maintenance treatments for stage IIIb/IV non-small-cell lung cancer (NSCLC) patients with good performance status not progressing after first-line induction chemotherapy: results by performance status, EGFR mutation, histology and response to previous induction. Eur J Cancer. 2015;51(16):2330–44. https://doi.org/10.1016/j.ejca.2015.07.007.
Crinò L. P3.02c-095 - Italian Nivolumab Expanded access programme: efficacy and safety data in squamous non small cell lung cancer patients. poster session with presenters, World Conference on Lung Cancer, PresentTrack: Advanced NSCLC, 2016.
Garassino MC, Gelibter AJ, Grossi F, Chiari R, Parra HS, Cascinu S, Cognetti F, Turci D, Blasi L, Bengala C, Mini E, Baldini EE, Quadrini S, Ceresoli GL, Antonelli P, Vasile E, Pinto C, Fasola G, Galetta D, Macerelli M, Giannarelli D, Lo Russo G, de Marinis F. Italian nivolumab expanded access program in nonsquamous non-small-cell lung cancer patients: results in never-smokers and EGFR-mutant patients. J Thorac Oncol. 2018. https://doi.org/10.1016/j.jtho.2018.04.025.
Yoo SH, Keam B, Kim M, Kim TM, Kim DW, Heo DS. Generalization and representativeness of phase III immune checkpoint blockade trials in non-small cell lung cancer. Thorac Cancer. 2018;9(6):736–44. https://doi.org/10.1111/1759-7714.12641.
Grossi F. 1156P Italian nivolumab expanded access programme: real-world results in non-squamous non-small cell lung cancer patients. European Society of Medical Oncology 2017 Congress. Ann Oncol. 2017;28(suppl_5):v403–27.
Funding
No funding to declare.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Bersanelli, M., Brighenti, M., Buti, S. et al. Patient performance status and cancer immunotherapy efficacy: a meta-analysis. Med Oncol 35, 132 (2018). https://doi.org/10.1007/s12032-018-1194-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-018-1194-4