Abstract
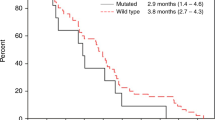
This clinical trial assessed the efficacy and toxicity of panitumumab combined with oxaliplatin and capecitabine as first-line treatment in KRAS exon 2 wild-type metastatic colorectal cancer (mCRC) patients. Patients with exon 2 KRAS wild-type mCRC received panitumumab 9 mg/Kg, oxaliplatin 130 mg/m2, and capecitabine 2000 mg/m2 repeated every 3 weeks. The primary endpoint was objective response rate (ORR, minimum 42 responses). We retrospectively assessed mutations in genes implicated in CRC with massively parallel sequencing; ERBB2 and EGFR amplification with fluorescence in situ hybridization, and tumor-infiltrating lymphocyte density. Among 78 patients enrolled, 45 (57.7%) completed 6 cycles. Most common grade 3–4 toxicities were skin rash (19.2%), diarrhea (18%), and neuropathy (6.4%). Among 5 (6.4%) potentially treatment-related deaths, 2 (2.6%) were characterized toxic. Objective response occurred in 43 (55.1%) of the patients (complete 6.4% and partial response 48.7%; stable 17.9% and progressive disease 7.7%), while 3.8% were non-evaluable and 15% discontinued their treatment early. Additional mutations in KRAS/NRAS/BRAF were found in 11/62 assessable (18%) tumors. After 51 months median follow-up, median progression-free (PFS) was 8.1 and overall survival 20.2 months, independently of KRAS/NRAS/BRAF or PI3K-pathway mutation status. Patients with TP53 mutations (n = 34; 55%), as well as those with left colon primary tumors (n = 66; 85%), had significantly better PFS, also confirmed in multivariate analysis. Although the clinical trial met its primary endpoint, according to the current standards, the efficacy and tolerability of the drug combination are considered insufficient. Extended genotyping yielded interesting results regarding the significance of TP53 mutations.
ClinicalTrials.gov identifier: NCT01215539, Registration date: Sep 29, 2010.




Similar content being viewed by others
References
Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490–502.
Ohhara Y, Fukuda N, Takeuchi S, et al. Role of targeted therapy in metastatic colorectal cancer. World J GastrointestOncol. 2016;8:642–55.
Holch JW, Ricard I, Stintzing S, et al. The relevance of primary tumour location in patients with metastatic colorectal cancer: a meta-analysis of first-line clinical trials. Eur J Cancer. 2017;70:87–98.
Petrelli F, Borgonovo K, Barni S. The predictive role of skin rash with cetuximab and panitumumab in colorectal cancer patients: a systematic review and meta-analysis of published trials. Target Oncol. 2013;8:173–81.
Kim JS, Lee C, Bonifant CL, et al. Activation of p53-dependent growth suppression in human cells by mutations in PTEN or PIK3CA. Mol Cell Biol. 2007;27:662–77.
EMA. EUROPEAN MEDICINES AGENCY: Vectibix Assessment Report. 2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000741/WC500148667.pdf. Accessed 23 April 2017.
Kotoula V, Charalambous E, Biesmans B, et al. Targeted KRAS mutation assessment on patient tumor histologic material in real time diagnostics. PLoS ONE. 2009;4:e7746.
Cancer Genome Atlas. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–7.
Montagut C, Dalmases A, Bellosillo B, et al. Identification of a mutation in the extracellular domain of the epidermal growth factor receptor conferring cetuximab resistance in colorectal cancer. Nat Med. 2012;18:221–3.
Pecot CV, Rupaimoole R, Yang D, et al. Tumour angiogenesis regulation by the miR-200 family. Nat Commun. 2013;4:2427.
McGranahan N, Favero F, de Bruin EC, et al. Clonal status of actionable driver events and the timing of mutational processes in cancer evolution. Sci Transl Med. 2015;7:283ra254.
Martin V, Landi L, Molinari F, et al. HER2 gene copy number status may influence clinical efficacy to anti-EGFR monoclonal antibodies in metastatic colorectal cancer patients. Br J Cancer. 2013;108:668–75.
Watters AD, Going JJ, Cooke TG, et al. Chromosome 17 aneusomy is associated with poor prognostic factors in invasive breast carcinoma. Breast Cancer Res Treat. 2003;77:109–14.
Huh JW, Lee JH, Kim HR. Prognostic significance of tumor-infiltrating lymphocytes for patients with colorectal cancer. Arch Surg. 2012;147:366–72.
Kirilovsky A, Marliot F, El Sissy C, et al. Rational bases for the use of the Immunoscore in routine clinical settings as a prognostic and predictive biomarker in cancer patients. Int Immunol. 2016;28:373–82.
Mascarello JT, Hirsch B, Kearney HM, et al. Section E9 of the American College of Medical Genetics technical standards and guidelines: fluorescence in situ hybridization. Genet Med. 2011;13:667–75.
De Roock W, Claes B, Bernasconi D, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11:753–62.
Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065–75.
Bokemeyer C, Bondarenko I, Hartmann JT, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol. 2011;22:1535–46.
Schwartzberg LS, Rivera F, Karthaus M, et al. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol. 2014;32:2240–7.
Van Cutsem E, Lenz HJ, Köhne CH, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol. 2015;33:692–700.
Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697–705.
Maughan TS, Adams RA, Smith CG, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377:2103–14.
Moosmann N, von Weikersthal LF, Vehling-Kaiser U, et al. Cetuximab plus capecitabine and irinotecan compared with cetuximab plus capecitabine and oxaliplatin as first-line treatment for patients with metastatic colorectal cancer: AIO KRK-0104-a randomized trial of the German AIO CRC study group. J Clin Oncol. 2011;29:1050–8.
Borner M, Koeberle D, Von Moos R, et al. Adding cetuximab to capecitabine plus oxaliplatin (XELOX) in first-line treatment of metastatic colorectal cancer: a randomized phase II trial of the Swiss Group for Clinical Cancer Research SAKK. Ann Oncol. 2008;19:1288–92.
Hazama S, Maeda H, Iwamoto S, et al. A phase II study of XELOX and cetuximab as first-line therapy in patients with KRAS wild type metastatic colorectal cancer (FLEET2 Study). Clin Colorectal Cancer. 2016;15:329–36.
Rachar V, Czejka M, Kitzmueller M, et al. Assessment of pharmacokinetic interaction between capecitabine and cetuximab in metastatic colorectal cancer patients. Anticancer Res. 2016;36:4715–23.
Kerr DJ, Domingo E, Kerr R. Is sidedness prognostically important across all stages of colorectal cancer? Lancet Oncol. 2016;17:1480–2.
Lee MS, Menter DG, Kopetz S. Right versus left colon cancer biology: integrating the consensus molecular subtypes. J Natl Compr Canc Netw. 2017;15:411–9.
Loupakis F, Yang D, Yau L, et al. Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer Inst. 2015;107(3):dju427.
Sunakawa Y, Ichikawa W, Tsuji A, et al. Prognostic impact of primary tumor location on clinical outcomes of metastatic colorectal cancer treated with cetuximab plus oxaliplatin-based chemotherapy: a subgroup analysis of the JACCRO CC-05/06 trials. Clin Colorectal Cancer. 2017;16:e171–e180.
Normanno N, Rachiglio AM, Lambiase M, et al. Heterogeneity of KRAS, NRAS, BRAF and PIK3CA mutations in metastatic colorectal cancer and potential effects on therapy in the CAPRI GOIM trial. Ann Oncol. 2015;26:1710–4.
Li XL, Zhou J, Chen ZR, et al. P53 mutations in colorectal cancer—molecular pathogenesis and pharmacological reactivation. World J Gastroenterol. 2015;21:84–93.
Vakiani E, Janakiraman M, Shen R, et al. Comparative genomic analysis of primary versus metastatic colorectal carcinomas. J Clin Oncol. 2012;30:2956–62.
Ciardiello F, Normanno N, Maiello E, et al. Clinical activity of FOLFIRI plus cetuximab according to extended gene mutation status by next-generation sequencing: findings from the CAPRI-GOIM trial. Ann Oncol. 2014;25:1756–61.
Russo A, Bazan V, Iacopetta B, et al. The TP53 colorectal cancer international collaborative study on the prognostic and predictive significance of p53 mutation: influence of tumor site, type of mutation, and adjuvant treatment. J Clin Oncol. 2005;23:7518–28.
Russo AL, Borger DR, Szymonifka J, et al. Mutational analysis and clinical correlation of metastatic colorectal cancer. Cancer. 2014;120:1482–90.
Oden-Gangloff A, Di Fiore F, Bibeau F, et al. TP53 mutations predict disease control in metastatic colorectal cancer treated with cetuximab-based chemotherapy. Br J Cancer. 2009;100:1330–5.
Huemer F, Thaler J, Piringer G, et al. Sidedness and TP53 mutations impact OS in anti-EGFR but not anti-VEGF treated mCRC—an analysis of the KRAS registry of the AGMT (Arbeitsgemeinschaft Medikamentöse Tumortherapie). BMC Cancer. 2018;18:11.
Schell MJ, Yang M, Teer JK, et al. A multigene mutation classification of 468 colorectal cancers reveals a prognostic role for APC. Nat Commun. 2016;7:11743.
Acknowledgements
The authors are indebted to all patients and their families for their trust and participation in the HE6A/09 trial as well as for the provision of biological material for research purposes. The authors wish to thank Dimitra Katsala for monitoring the study, Athina Goudopoulou for clinical safety coordination, and Maria Moschoni for data coordination.
Funding
The study was supported by a research grant from Amgen Ltd. and by an internal Hellenic Cooperative Oncology Group (HeCOG) translational research grant (HER_6A/09).
Author information
Authors and Affiliations
Contributions
GPap conceived the study, participated in its design, contributed to the acquisition, analysis, and interpretation of data, and drafted the manuscript. VKot conceived the study, participated in its design, contributed to the acquisition, analysis, and interpretation of data and drafted the manuscript. EG contributed to the analysis and interpretation of data and revised critically the manuscript. GAK contributed to the analysis and interpretation of data and drafted the manuscript. VKar contributed to the acquisition of data and revised critically the manuscript. SL contributed to the acquisition, analysis, and interpretation of data and revised critically the manuscript. AKou contributed to the acquisition of data and revised critically the manuscript. MB contributed to the acquisition, analysis, and interpretation of data and revised critically the manuscript. EC contributed to the acquisition, analysis, and interpretation of data and revised critically the manuscript. ED contributed to the acquisition, analysis, and interpretation of data and revised critically the manuscript. KC contributed to the acquisition of data and revised critically the manuscript. GT contributed to the acquisition of data and revised critically the manuscript. EP contributed to the acquisition of data and revised critically the manuscript. SC contributed to the acquisition, analysis, and interpretation of data and revised critically the manuscript. ES contributed to the acquisition of data and revised critically the manuscript. IGK contributed to the acquisition of data and revised critically the manuscript. IV contributed to the acquisition of data and revised critically the manuscript. AKon contributed to the acquisition of data and revised critically the manuscript. KNS contributed to the acquisition of data and revised critically the manuscript. GPen conceived the study, participated in its design, contributed to the acquisition, analysis, and interpretation of data, and drafted the manuscript. DP conceived the study, participated in its design, contributed to the acquisition of data, and drafted the manuscript. GF conceived the study, participated in its design, contributed to the acquisition, analysis, and interpretation of data, and drafted the manuscript. All authors approved the final version of the manuscript to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
VKar: Advisory Board of Amgen, Pfizer, Novartis, BI, Lilly, Astellas, Genesis-Pharma, and Janssen. SL: Employee of NEO New Oncology GmbH and Consultant BioTech AG. ES: Advisory Board of Merck, MSD, Astra-Zeneca, Roche, Amgen, and Genesis. GF: Advisory Board of Pfizer, Sanofi, and Roche. Honoraria from Astra-Zeneca. The rest of the authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The clinical protocol was approved by the Institutional Review Boards of Papageorgiou General Hospital, Ioannina University Hospital, Agii Anargiri Cancer Hospital, Sotiria General Hospital, Alexandra Hospital, Patra University Hospital, Metropolitan Hospital, Hygeia Hospital, Chania General Hospital, Hippokration Hospital, and by the National Organization for Medicines. The trial was registered with the ClinicalTrials.gov identifier: NCT01215539. The translational research protocol was approved by the Institutional Review Board of the Papageorgiou General Hospital (08/04/2009 #233).
Informed consent
Informed consent was obtained from all individual participants included in the study. In addition, patients who were willing to provide biological material for future translational research studies signed a separate informed consent.
Additional information
George Papaxoinis and Vassiliki Kotoula authors contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Papaxoinis, G., Kotoula, V., Giannoulatou, E. et al. Phase II study of panitumumab combined with capecitabine and oxaliplatin as first-line treatment in metastatic colorectal cancer patients: clinical results including extended tumor genotyping. Med Oncol 35, 101 (2018). https://doi.org/10.1007/s12032-018-1160-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-018-1160-1