Abstract
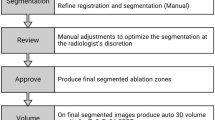
Liver thermal ablation is an alternative treatment for hepatocellular carcinoma (HCC) and secondary liver malignancies. Microwave ablation (MWA) produces large ablation zones (AZ) in short time; however, AZ prediction is based on preclinical ex vivo models, rising concerns about reproducibility and safety in humans. We aimed to investigate the effects produced by a new-generation MWA system on human liver in vivo with different approaches (percutaneous or intraoperative) and liver conditions (cirrhosis or previous chemotherapy treatment), in comparison with manufacturer-provided predictions based on ex vivo animal models. Complete tumor ablation (CA) and early clinical outcomes were also assessed. From October 2014, 60 consecutive patients (cirrhotic = 31; non-cirrhotic = 10; chemotherapy-treated = 19) with 81 liver nodules (HCC = 31; mets = 50) underwent MWA procedures (percutaneous = 30; laparotomic = 18; laparoscopic = 12), with a 2450 MHz/100 W generator with Thermosphere™ Technology (Emprint™, Medtronic). A contrast-enhanced CT or MR was performed after one month to assess CA and measure AZ. A linear correlation between AZ volumes and ablation times was observed in vivo, without differences from manufacturer-provided ex vivo predictions in all operative approaches and liver conditions. Other independent variables (sex, age, nodule location) showed no relationship when added to the model. Median (IQR) longitudinal and transverse roundness-indexes of the AZs were, respectively, 0.77(0.13) and 0.93(0.11). CA at 1 month was 93% for percutaneous and 100% for intraoperative procedures (p = 0.175). Thirty-day morbidity and mortality were 3% and 0%. MWA with Thermosphere™ Technology produces predictable AZs on human liver in vivo, according to manufacturer-provided ex vivo predictions. In our experience, this new-generation MWA system is effective and safe to treat liver malignancies in different operative and clinical settings.

Similar content being viewed by others
References
Weis S, Franke A, Mossner J, Jakobsen JC, Schoppmeyer K. Radiofrequency (thermal) ablation versus no intervention or other interventions for hepatocellular carcinoma. Cochrane Database Systematic Rev. 2013;(12):CD003046. doi:10.1002/14651858.CD003046.pub3.
Cirocchi R, Trastulli S, Boselli C, Montedori A, Cavaliere D, Parisi A, et al. Radiofrequency ablation in the treatment of liver metastases from colorectal cancer. Cochrane Database Systematic Rev. 2012;(6):CD006317. doi:10.1002/14651858.CD006317.pub3.
Wong SL, Mangu PB, Choti MA, Crocenzi TS, Dodd GD 3rd, Dorfman GS, et al. American society of clinical oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2010;28(3):493–508. doi:10.1200/JCO.2009.23.4450.
European Association for the Study of the L, European Organisation for R, Treatment of C. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–43. doi:10.1016/j.jhep.2011.12.001.
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. doi:10.1002/ijc.29210.
Lencioni R, Cioni D, Crocetti L, Franchini C, Pina CD, Lera J, et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234(3):961–7. doi:10.1148/radiol.2343040350.
Liu PH, Hsu CY, Hsia CY, Lee YH, Huang YH, Chiou YY, et al. Surgical resection versus radiofrequency ablation for single hepatocellular carcinoma ≤ 2 cm in a propensity score model. Ann Surg. 2016;263(3):538–45. doi:10.1097/SLA.0000000000001178.
Yamakado K, Nakatsuka A, Takaki H, Yokoi H, Usui M, Sakurai H, et al. Early-stage hepatocellular carcinoma: radiofrequency ablation combined with chemoembolization versus hepatectomy. Radiology. 2008;247(1):260–6. doi:10.1148/radiol.2471070818.
Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243(3):321–8. doi:10.1097/01.sla.0000201480.65519.b8.
Hou YF, Wei YG, Yang JY, Wen TF, Xu MQ, Yan LN, et al. Combined hepatectomy and radiofrequency ablation versus TACE in improving survival of patients with unresectable BCLC stage B HCC. Hepatobiliary Pancreat Dis Int HBPD INT. 2016;15(4):378–85.
Choi D, Lim HK, Joh JW, Kim SJ, Kim MJ, Rhim H, et al. Combined hepatectomy and radiofrequency ablation for multifocal hepatocellular carcinomas: long-term follow-up results and prognostic factors. Ann Surg Oncol. 2007;14(12):3510–8. doi:10.1245/s10434-007-9492-7.
van Amerongen MJ, van der Stok EP, Futterer JJ, Jenniskens SF, Moelker A, Grunhagen DJ, et al. Short term and long term results of patients with colorectal liver metastases undergoing surgery with or without radiofrequency ablation. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2016;42(4):523–30. doi:10.1016/j.ejso.2016.01.013.
Philips P, Groeschl RT, Hanna EM, Swan RZ, Turaga KK, Martinie JB, et al. Single-stage resection and microwave ablation for bilobar colorectal liver metastases. Br J Surg. 2016;103(8):1048–54. doi:10.1002/bjs.10159.
Wada Y, Takami Y, Tateishi M, Ryu T, Mikagi K, Saitsu H. Efficacy of surgical treatment using microwave coagulo-necrotic therapy for unresectable multiple colorectal liver metastases. OncoTargets Ther. 2016;9:937–43. doi:10.2147/OTT.S97824.
Yang PC, Lin BR, Chen YC, Lin YL, Lai HS, Huang KW, et al. Local control by radiofrequency thermal ablation increased overall survival in patients with refractory liver metastases of colorectal cancer. Medicine. 2016;95(14):e3338. doi:10.1097/MD.0000000000003338.
Nielsen K, Scheffer HJ, Volders JH, van der Vorst MJ, van Tilborg AA, Comans EF, et al. Radiofrequency ablation to improve survival after conversion chemotherapy for colorectal liver metastases. World J Surg. 2016;40(8):1951–8. doi:10.1007/s00268-016-3554-6.
Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg SN. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265(3):958–68. doi:10.1148/radiol.12111851.
Weng M, Zhang Y, Zhou D, Yang Y, Tang Z, Zhao M, et al. Radiofrequency ablation versus resection for colorectal cancer liver metastases: a meta-analysis. PLoS ONE. 2012;7(9):e45493. doi:10.1371/journal.pone.0045493.
Gillams A, Goldberg N, Ahmed M, Bale R, Breen D, Callstrom M, et al. Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, the Interventional Oncology Sans Frontieres meeting 2013. Eur Radiol. 2015;25(12):3438–54. doi:10.1007/s00330-015-3779-z.
Wells SA, Hinshaw JL, Lubner MG, Ziemlewicz TJ, Brace CL, Lee FT Jr. Liver ablation: best practice. Radiol Clin North Am. 2015;53(5):933–71. doi:10.1016/j.rcl.2015.05.012.
Barral M, Auperin A, Hakime A, Cartier V, Tacher V, Otmezguine Y, et al. Percutaneous thermal ablation of breast cancer metastases in oligometastatic patients. Cardiovasc Interv Radiol. 2016;39(6):885–93. doi:10.1007/s00270-016-1301-x.
Meloni MF, Andreano A, Laeseke PF, Livraghi T, Sironi S, Lee FT Jr. Breast cancer liver metastases: US-guided percutaneous radiofrequency ablation—intermediate and long-term survival rates. Radiology. 2009;253(3):861–9. doi:10.1148/radiol.2533081968.
Guner A, Son T, Cho I, Kwon IG, An JY, Kim HI, et al. Liver-directed treatments for liver metastasis from gastric adenocarcinoma: comparison between liver resection and radiofrequency ablation. Gastric Cancer Off J Int Gastric Cancer Assoc Jpn Gastric Cancer Assoc. 2016;19(3):951–60. doi:10.1007/s10120-015-0522-z.
Velez E, Goldberg SN, Kumar G, Wang Y, Gourevitch S, Sosna J, et al. Hepatic thermal ablation: effect of device and heating parameters on local tissue reactions and distant tumor growth. Radiology. 2016;281(3):782–92. doi:10.1148/radiol.2016152241.
Tannous BA, Teng J. Secreted blood reporters: insights and applications. Biotechnol Adv. 2011;29(6):997–1003. doi:10.1016/j.biotechadv.2011.08.021.
Kim KR, Thomas S. Complications of image-guided thermal ablation of liver and kidney neoplasms. Semin Interv Radiol. 2014;31(2):138–48. doi:10.1055/s-0034-1373789.
Lee KF, Wong J, Hui JW, Cheung YS, Chong CC, Fong AK, et al. Long-term outcomes of microwave versus radiofrequency ablation for hepatocellular carcinoma by surgical approach: a retrospective comparative study. Asian J Surg. 2016;. doi:10.1016/j.asjsur.2016.01.001.
Poulou LS, Botsa E, Thanou I, Ziakas PD, Thanos L. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol. 2015;7(8):1054–63. doi:10.4254/wjh.v7.i8.1054.
Ierardi AM, Mangano A, Floridi C, Dionigi G, Biondi A, Duka E, et al. A new system of microwave ablation at 2450 MHz: preliminary experience. Updates Surg. 2015;67(1):39–45. doi:10.1007/s13304-015-0288-1.
Ierardi AM, Giorlando F, Piacentino F, Fontana F, Novario R, Angileri SA, Duka E, Carrafiello G. Factors predicting outcomes of microwave ablation of small hepatocellular carcinoma. Radiol Med. 2016. doi:10.1007/s11547-016-0694-6.
Ierardi AM, Floridi C, Fontana F, Chini C, Giorlando F, Piacentino F, Brunese L, Pinotti G, Bacuzzi A, Carrafiello G. Microwave ablation of liver metastases to overcome the limitation of radiofrequency ablation. Radiol Med. 2013;118(6):949–61. doi:10.1007/s11547-013-0968-1.
Yun D, Kim S, Song I, Chun K. Comparative analysis of Laparoscopic versus open surgical radiofrequency ablation for malignant liver tumors. Korean J Hepato-Biliary-Pancreat Surg. 2014;18(4):122–8. doi:10.14701/kjhbps.2014.18.4.122.
Casaccia M, Andorno E, Nardi I, Troilo B, Barabino G, Santori G, et al. Laparoscopic US-guided radiofrequency ablation of unresectable hepatocellular carcinoma in liver cirrhosis: feasibility and clinical outcome. J Laparoendosc Adv Surg Techn Part A. 2008;18(6):797–801. doi:10.1089/lap.2008.0039.
Chiang J, Wang P, Brace CL. Computational modelling of microwave tumour ablations. Int J Hyperth Off J Eur Soc Hyperth Oncol N Am Hyperthe Group. 2013;29(4):308–17. doi:10.3109/02656736.2013.799295.
Shyn PB, Bird JR, Koch RM, Tatli S, Levesque VM, Catalano PJ, et al. Hepatic microwave ablation zone size: correlation with total energy, net energy, and manufacturer-provided chart predictions. J Vasc Interv Radiol JVIR. 2016;27(9):1389–96. doi:10.1016/j.jvir.2016.05.009.
Siriwardana PN, Singh S, Johnston EW, Watkins J, Bandula S, Illing RO, et al. Effect of hepatic perfusion on microwave ablation zones in an ex vivo porcine liver model. J Vasc Interv Radiol JVIR. 2016;. doi:10.1016/j.jvir.2016.03.006.
Amabile C, Ahmed M, Solbiati L, Meloni MF, Solbiati M, Cassarino S, et al. Microwave ablation of primary and secondary liver tumours: ex vivo, in vivo, and clinical characterisation. Int J Hyperth Off J Eur Soc Hyperth Oncol N Am Hyperthe Group. 2016;. doi:10.1080/02656736.2016.1196830.
Wang LG, Jiang WJ, Fan WJ, Zheng YB, Song XP, Liu S, et al. Microwave ablation: the differences between biliary cirrhosis and normal porcine liver using a cooled-tip electrode. Anticancer Res. 2016;36(3):1221–6.
Wang X, Sofocleous CT, Erinjeri JP, Petre EN, Gonen M, Do KG, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Interv Radiol. 2013;36(1):166–75. doi:10.1007/s00270-012-0377-1.
Shady W, Petre EN, Gonen M, Erinjeri JP, Brown KT, Covey AM, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes—a 10-year experience at a single center. Radiology. 2016;278(2):601–11. doi:10.1148/radiol.2015142489.
Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. The National Cancer Institute, Bethesda Approved after October 1, 2009; 2010.
Liu D, Brace CL. CT imaging during microwave ablation: analysis of spatial and temporal tissue contraction. Med Phys. 2014;41(11):113303. doi:10.1118/1.4897381.
Bouda D, Lagadec M, Alba CG, Barrau V, Burgio MD, Moussa N, et al. Imaging review of hepatocellular carcinoma after thermal ablation: the good, the bad, and the ugly. J Magn Reson Imaging JMRI. 2016;44(5):1070–90. doi:10.1002/jmri.25369.
Lencioni R, de Baere T, Martin RC, Nutting CW, Narayanan G. Image-guided ablation of malignant liver tumors: recommendations for clinical validation of novel thermal and non-thermal technologies—a western perspective. Liver Cancer. 2015;4(4):208–14. doi:10.1159/000367747.
Sotirchos VS, Petrovic LM, Gonen M, Klimstra DS, Do RK, Petre EN, et al. Colorectal cancer liver metastases: biopsy of the ablation zone and margins can be used to predict oncologic outcome. Radiology. 2016;280(3):949–59. doi:10.1148/radiol.2016151005.
Poggi G, Tosoratti N, Montagna B, Picchi C. Microwave ablation of hepatocellular carcinoma. World J Hepatol. 2015;7(25):2578–89. doi:10.4254/wjh.v7.i25.2578.
Nafidi O, Desy D, Letourneau R, Cote J, Plasse M, Vandenbroucke F, et al. Hypertrophy of the non-embolized liver after chemotherapy. HPB Off J Int Hepato Pancreato Biliary Assoc. 2009;11(2):103–7. doi:10.1111/j.1477-2574.2009.00004.x.
Rubbia-Brandt L, Audard V, Sartoretti P, Roth AD, Brezault C, Le Charpentier M, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol Off J Eur Soc Med Oncol. 2004;15(3):460–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
De Cobelli, F., Marra, P., Ratti, F. et al. Microwave ablation of liver malignancies: comparison of effects and early outcomes of percutaneous and intraoperative approaches with different liver conditions. Med Oncol 34, 49 (2017). https://doi.org/10.1007/s12032-017-0903-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-017-0903-8