Abstract
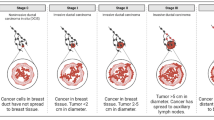
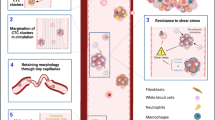
Circulating tumor cells (CTC) in the blood of cancer patients are regarded as potential metastatic seeds, and their detailed characterization holds great promises for more exact prognosis, better diagnosis and therapy of the metastatic cancer. Circulating tumor cell clusters represent different class of CTCs, with specific properties, including high metastatic potential. In this review, we present current opinions on differences between single CTCs and CTC clusters, their mode of dissemination, methods of detection and clinical importance in various types of cancer.

Similar content being viewed by others
Abbreviations
- CTC:
-
Circulating tumor cells
- EMT:
-
Epithelial–mesenchymal transition
- MET:
-
Mesenchymal–epithelial transition
- ISET:
-
Isolation by size of epithelial tumor cells
- FMSA:
-
Flexible micro spring array
- FDA:
-
Food and Drug Administration
- MBC:
-
Metastatic breast cancer
- MCA:
-
Microcavity array
- CEA:
-
Carcinoembryonic antigen
- CK:
-
Cytokeratins
- CTM:
-
Circulating tumor microemboli
- TGF-β:
-
Transforming growth factor β
- EpCAM:
-
Epithelial adhesion molecule
- DDF:
-
Dean flow fractionation
- HER2:
-
Human epidermal growth factor
- mCRPC:
-
Metastatic castration-resistant prostate cancer
- DAPI:
-
4′,6-diamidino-2-phenylindole
- IF:
-
Immunofluorescence
- PFS:
-
Progression-free survival
- OS:
-
Overall survival
- NSCLC:
-
Non-small cell lung cancer
- FU:
-
Follow-up
- ccRCC:
-
Clear cell renal cell carcinoma
- PDAC:
-
Pancreatic ductal adenocarcinoma
References
Massague J, Obenauf AC. Metastatic colonization by circulating tumour cells. Nature. 2016;529(7586):298–306. doi:10.1038/nature17038.
Ashworth T. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Aust Med J. 1869;14(3):146–9.
Watanabe S. The metastasizability of tumor cells. Cancer. 1954;7(2):215–23.
Paget G. Remarks on a case of alternate partial anaesthesia. Br Med J. 1889;1(1462):1–3.
Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315(26):1650–9. doi:10.1056/NEJM198612253152606.
Duda DG, Duyverman AM, Kohno M, Snuderl M, Steller EJ, Fukumura D, et al. Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci USA. 2010;107(50):21677–82. doi:10.1073/pnas.1016234107.
Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158(5):1110–22. doi:10.1016/j.cell.2014.07.013.
Aceto N, Toner M, Maheswaran S, Haber DA. En route to metastasis: circulating tumor cell clusters and epithelial-to-mesenchymal transition. Trends Cancer. 2015;1(1):44–52. doi:10.1016/j.trecan.2015.07.006.
Hong Y, Fang F, Zhang Q. Circulating tumor cell clusters: what we know and what we expect (review). Int J Oncol. 2016;. doi:10.3892/ijo.2016.3747.
Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339(6119):580–4. doi:10.1126/science.1228522.
Cheung KJ, Padmanaban V, Silvestri V, Schipper K, Cohen JD, Fairchild AN, et al. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc Natl Acad Sci USA. 2016;113(7):E854–63. doi:10.1073/pnas.1508541113.
Au SH, Storey BD, Moore JC, Tang Q, Chen YL, Javaid S, et al. Clusters of circulating tumor cells traverse capillary-sized vessels. Proc Natl Acad Sci USA. 2016;113(18):4947–52. doi:10.1073/pnas.1524448113.
Koren S, Bentires-Alj M. Breast tumor heterogeneity: source of Fitness. Hurdle for Therapy. Mol Cell. 2015;60(4):537–46. doi:10.1016/j.molcel.2015.10.031.
Maddipati R, Stanger BZ. Pancreatic cancer metastases harbor evidence of polyclonality. Cancer Discov. 2015;5(10):1086–97. doi:10.1158/2159-8290.CD-15-0120.
Thiery JP. Epithelial–mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–54. doi:10.1038/nrc822.
Fischer KR, Durrans A, Lee S, Sheng J, Li F, Wong ST, et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527(7579):472–6. doi:10.1038/nature15748.
Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial–mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20(5):576–90. doi:10.1016/j.ccr.2011.09.009.
Cima I, Kong SL, Sengupta D, Tan IB, Phyo WM, Lee D, et al. Tumor-derived circulating endothelial cell clusters in colorectal cancer. Sci Transl Med. 2016;8(345):345ra89. doi:10.1126/scitranslmed.aad7369.
Cheung KJ, Ewald AJ. A collective route to metastasis: seeding by tumor cell clusters. Science. 2016;352(6282):167–9. doi:10.1126/science.aaf6546.
Alunni-Fabbroni M, Sandri MT. Circulating tumour cells in clinical practice: methods of detection and possible characterization. Methods. 2010;50(4):289–97. doi:10.1016/j.ymeth.2010.01.027.
Harouaka R, Kang Z, Zheng SY, Cao L. Circulating tumor cells: advances in isolation and analysis, and challenges for clinical applications. Pharmacol Ther. 2014;141(2):209–21. doi:10.1016/j.pharmthera.2013.10.004.
Mohamed H, Murray M, Turner JN, Caggana M. Isolation of tumor cells using size and deformation. J Chromatogr A. 2009;1216(47):8289–95. doi:10.1016/j.chroma.2009.05.036.
Tan SJ, Yobas L, Lee GY, Ong CN, Lim CT. Microdevice for the isolation and enumeration of cancer cells from blood. Biomed Microdevices. 2009;11(4):883–92. doi:10.1007/s10544-009-9305-9.
Zheng S, Lin H, Liu JQ, Balic M, Datar R, Cote RJ, et al. Membrane microfilter device for selective capture, electrolysis and genomic analysis of human circulating tumor cells. J Chromatogr A. 2007;1162(2):154–61. doi:10.1016/j.chroma.2007.05.064.
Hou HW, Warkiani ME, Khoo BL, Li ZR, Soo RA, Tan DS, et al. Isolation and retrieval of circulating tumor cells using centrifugal forces. Sci Rep. 2013;3:1259. doi:10.1038/srep01259.
Harouaka RA, Zhou MD, Yeh YT, Khan WJ, Das A, Liu X, et al. Flexible micro spring array device for high-throughput enrichment of viable circulating tumor cells. Clin Chem. 2014;60(2):323–33. doi:10.1373/clinchem.2013.206805.
Rosenberg R, Gertler R, Friederichs J, Fuehrer K, Dahm M, Phelps R, et al. Comparison of two density gradient centrifugation systems for the enrichment of disseminated tumor cells in blood. Cytometry. 2002;49(4):150–8. doi:10.1002/cyto.10161.
Gertler R, Rosenberg R, Fuehrer K, Dahm M, Nekarda H, Siewert JR. Detection of circulating tumor cells in blood using an optimized density gradient centrifugation. Recent Results Cancer Res. 2003;162:149–55.
Naume B, Borgen E, Tossvik S, Pavlak N, Oates D, Nesland JM. Detection of isolated tumor cells in peripheral blood and in BM: evaluation of a new enrichment method. Cytotherapy. 2004;6(3):244–52. doi:10.1080/14653240410006086.
McNiece I, Briddell R, Stoney G, Kern B, Zilm K, Recktenwald D, et al. Large-scale isolation of CD34+ cells using the Amgen cell selection device results in high levels of purity and recovery. J Hematother. 1997;6(1):5–11. doi:10.1089/scd.1.1997.6.5.
Neurauter AA, Bonyhadi M, Lien E, Nokleby L, Ruud E, Camacho S, et al. Cell isolation and expansion using Dynabeads. Adv Biochem Eng Biotechnol. 2007;106:41–73. doi:10.1007/10_2007_072.
Peters CE, Woodside SM, Eaves AC. Isolation of subsets of immune cells. Methods Mol Biol. 2005;302:95–116. doi:10.1385/1-59259-903-6:095.
Lankiewicz S, Rivero BG, Bocher O. Quantitative real-time RT-PCR of disseminated tumor cells in combination with immunomagnetic cell enrichment. Mol Biotechnol. 2006;34(1):15–27. doi:10.1385/MB:34:1:15.
Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351(8):781–91. doi:10.1056/NEJMoa040766.
Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(19):3213–21. doi:10.1200/JCO.2007.15.8923.
Danila DC, Heller G, Gignac GA, Gonzalez-Espinoza R, Anand A, Tanaka E, et al. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res. 2007;13(23):7053–8. doi:10.1158/1078-0432.CCR-07-1506.
Cristofanilli M, Hayes DF, Budd GT, Ellis MJ, Stopeck A, Reuben JM, et al. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol. 2005;23(7):1420–30. doi:10.1200/JCO.2005.08.140.
Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9(4):265–73. doi:10.1038/nrc2620.
Jansson S, Bendahl PO, Larsson AM, Aaltonen KE, Ryden L. Prognostic impact of circulating tumor cell apoptosis and clusters in serial blood samples from patients with metastatic breast cancer in a prospective observational cohort. BMC Cancer. 2016;16:433. doi:10.1186/s12885-016-2406-y.
Mu Z, Wang C, Ye Z, Austin L, Civan J, Hyslop T, et al. Prospective assessment of the prognostic value of circulating tumor cells and their clusters in patients with advanced-stage breast cancer. Breast Cancer Res Treat. 2015;154(3):563–71. doi:10.1007/s10549-015-3636-4.
Paoletti C, Li Y, Muniz MC, Kidwell KM, Aung K, Thomas DG, et al. Significance of circulating tumor cells in metastatic triple-negative breast cancer patients within a randomized, phase II trial: TBCRC 019. Clin Cancer Res. 2015;21(12):2771–9. doi:10.1158/1078-0432.CCR-14-2781.
Hou JM, Krebs MG, Lancashire L, Sloane R, Backen A, Swain RK, et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol. 2012;30(5):525–32. doi:10.1200/JCO.2010.33.3716.
Stott SL, Hsu CH, Tsukrov DI, Yu M, Miyamoto DT, Waltman BA, et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc Natl Acad Sci USA. 2010;107(43):18392–7. doi:10.1073/pnas.1012539107.
Hosokawa M, Yoshikawa T, Negishi R, Yoshino T, Koh Y, Kenmotsu H, et al. Microcavity array system for size-based enrichment of circulating tumor cells from the blood of patients with small-cell lung cancer. Anal Chem. 2013;85(12):5692–8. doi:10.1021/ac400167x.
Hosokawa M, Kenmotsu H, Koh Y, Yoshino T, Yoshikawa T, Naito T, et al. Size-based isolation of circulating tumor cells in lung cancer patients using a microcavity array system. PLoS One. 2013;8(6):e67466. doi:10.1371/journal.pone.0067466.
Molnar B, Ladanyi A, Tanko L, Sreter L, Tulassay Z. Circulating tumor cell clusters in the peripheral blood of colorectal cancer patients. Clin Cancer Res. 2001;7(12):4080–5.
McDaniel AS, Ferraldeschi R, Krupa R, Landers M, Graf R, Louw J, et al. Phenotypic diversity of circulating tumour cells in patients with metastatic castration-resistant prostate cancer. BJU Int. 2016;. doi:10.1111/bju.13631.
Luo X, Mitra D, Sullivan RJ, Wittner BS, Kimura AM, Pan S, et al. Isolation and molecular characterization of circulating melanoma cells. Cell Rep. 2014;7(3):645–53. doi:10.1016/j.celrep.2014.03.039.
Sarioglu AF, Aceto N, Kojic N, Donaldson MC, Zeinali M, Hamza B, et al. A microfluidic device for label-free, physical capture of circulating tumor cell clusters. Nat Methods. 2015;12(7):685–91. doi:10.1038/nmeth.3404.
Cho EH, Wendel M, Luttgen M, Yoshioka C, Marrinucci D, Lazar D, et al. Characterization of circulating tumor cell aggregates identified in patients with epithelial tumors. Phys Biol. 2012;9(1):016001. doi:10.1088/1478-3975/9/1/016001.
Kats-Ugurlu G, Roodink I, de Weijert M, Tiemessen D, Maass C, Verrijp K, et al. Circulating tumour tissue fragments in patients with pulmonary metastasis of clear cell renal cell carcinoma. J Pathol. 2009;219(3):287–93. doi:10.1002/path.2613.
Krebs MG, Hou JM, Sloane R, Lancashire L, Priest L, Nonaka D, et al. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J Thorac Oncol. 2012;7(2):306–15. doi:10.1097/JTO.0b013e31823c5c16.
Liu MC, Shields PG, Warren RD, Cohen P, Wilkinson M, Ottaviano YL, et al. Circulating tumor cells: a useful predictor of treatment efficacy in metastatic breast cancer. J Clin Oncol. 2009;27(31):5153–9. doi:10.1200/JCO.2008.20.6664.
Molnar B, Floro L, Sipos F, Toth B, Sreter L, Tulassay Z. Elevation in peripheral blood circulating tumor cell number correlates with macroscopic progression in UICC stage IV colorectal cancer patients. Dis Markers. 2008;24(3):141–50.
Chang MC, Chang YT, Chen JY, Jeng YM, Yang CY, Tien YW, et al. Clinical significance of circulating tumor microemboli as a prognostic marker in patients with pancreatic ductal adenocarcinoma. Clin Chem. 2016;62(3):505–13. doi:10.1373/clinchem.2015.248260.
Acknowledgements
This study was funded by the National Science Centre (Grant 2014/14/M/NZ1/00437).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Anna Fabisiewicz declares that she has no conflict of interest. Ewa Grzybowska declares that she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Anna Fabisiewicz and Ewa Grzybowska contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Fabisiewicz, A., Grzybowska, E. CTC clusters in cancer progression and metastasis. Med Oncol 34, 12 (2017). https://doi.org/10.1007/s12032-016-0875-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-016-0875-0