Abstract
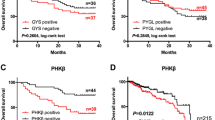
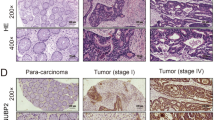
Prefoldin (PFDN) subunits have been reported upregulated in various tumor types, while the expression and functions of PFDN1 (PFDN subunit 1) in colorectal cancer (CRC) are not well elucidated. The aim of this study was to investigate the use of PFDN1 as a poor prognosis indicator for CRC and explore the functions of PFDN1 in CRC. The relationship between PFDN1 expression and CRC clinical-pathological statistics was detected on the tissue microarray containing 145 cases of CRC. ShRNA was used to silence PFDN1 expression in SW480 and RKO CRC cells, and these transfected cells were analyzed for changes in proliferation, colony formation, cell cycle, migration, and invasion. Immunofluorescence and immunoblot were used to determine the remodeling of the F-actin and α-tubulin. Finally, tumor growth on nude mice was observed and measured. In this study, we found PFDN1 was upregulated in CRC tissues compared with adjacent normal tissues. Also, PFDN1 expression positively correlated with tumor size and tumor invasion. Moreover, after silencing PFDN1 in SW480 and RKO cells, the proliferation and motility of CRC cells were significantly suppressed. The inhibitory effect of PFDN1 on tumor cell growth and motility was partially due to G2/M cell cycle blockage and cytoskeletal deficiency. Finally, in vivo assay showed that downregulation of PFDN1 inhibited tumor growth on nude mice and PFDN1 expression correlated with higher levels of Ki-67 staining. These findings indicate that PFDN1 was involved in the progression of CRC, and provide new insights into PFDN1 as a potential therapeutic target for CRC treatment.






Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90.
Sun JK, Li X, Xing JJ, Cao F, Wang H, Gong HF, Zhang W. Lentivirus-mediated silencing of USO1 inhibits cell proliferation and migration of human colon cancer cells. Med Oncol. 2015;32:218.
Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18(3):581–92.
Zhao H, Dong T, Zhou H, Wang L, Huang A, Feng B, Quan Y, Jin R, Zhang W, Sun J, Zhang D, Zheng M. miR-320a suppresses colorectal cancer progression by targeting Rac1. Carcinogenesis. 2014;35(4):886–95.
Qian-Lin Z, Ting-Feng W, Qi-Feng C, Min-Hua Z, Ai-Guo L. Inhibition of cytosolic chaperonin CCTζ-1 expression depletes proliferation of colorectal carcinoma in vitro. J Surg Oncol. 2010;102(5):419–23.
Huang X, Wang X, Cheng C, Cai J, He S, Wang H, Liu F, Zhu C, Ding Z, Huang X, Zhang T, Zhang Y. Chaperonin containing TCP1, subunit 8 (CCT8) is upregulated in hepatocellular carcinoma and promotes HCC proliferation. APMIS. 2014;122(11):1070–9.
Yokota S, Yamamoto Y, Shimizu K, Momoi H, Kamikawa T, Yamaoka Y, Yanagi H, Yura T, Kubota H. Increased expression of cytosolic chaperonin CCT in human hepatocellular and colonic carcinoma. Cell Stress Chaperones. 2001;6(4):345–50.
Siegers K, Waldmann T, Leroux MR, Grein K, Shevchenko A, Schiebel E, Hartl FU. Compartmentation of protein folding in vivo: sequestration of non-native polypeptide by the chaperonin-GimC system. EMBO J. 1999;18(1):75–84.
Siegert R, Leroux MR, Scheufler C, Hartl FU, Moarefi I. Structure of the molecular chaperone prefoldin: unique interaction of multiple coiled coil tentacles with unfolded proteins. Cell. 2000;103(4):621–32.
Martin-Benito J, Gomez-Reino J, Stirling PC, Lundin VF, Gomez-Puertas P, Boskovic J, Chacon P, Fernandez JJ, Berenguer J, Leroux MR, Valpuesta JM. Divergent substrate-binding mechanisms reveal an evolutionary specialization of eukaryotic prefoldin compared to its archaeal counterpart. Structure. 2007;15(1):101–10.
Hartl FU, Hayer-Hartl M. Converging concepts of protein folding in vitro and in vivo. Nat Struct Mol Biol. 2009;16(6):574–81.
Alldinger I, Dittert D, Peiper M, Fusco A, Chiappetta G, Staub E, Lohr M, Jesnowski R, Baretton G, Ockert D, Saeger HD, Grutzmann R, Pilarsky C. Gene expression analysis of pancreatic cell lines reveals genes overexpressed in pancreatic cancer. Pancreatology. 2005;5(4–5):370–9.
Cimmino F, Spano D, Capasso M, Zambrano N, Russo R, Zollo M, Iolascon A. Comparative proteomic expression profile in all-trans retinoic acid differentiated neuroblastoma cell line. J Proteome Res. 2007;6(7):2550–64.
Hansen WJ, Cowan NJ, Welch WJ. Prefoldin-nascent chain complexes in the folding of cytoskeletal proteins. J Cell Biol. 1999;145(2):265–77.
Martin-Benito J, Boskovic J, Gomez-Puertas P, Carrascosa JL, Simons CT, Lewis SA, Bartolini F, Cowan NJ, Valpuesta JM. Structure of eukaryotic prefoldin and of its complexes with unfolded actin and the cytosolic chaperonin CCT. EMBO J. 2002;21(23):6377–86.
Brackley KI, Grantham J. Activities of the chaperonin containing TCP-1 (CCT): implications for cell cycle progression and cytoskeletal organisation. Cell Stress Chaperones. 2009;14(1):23–31.
Coghlin C, Carpenter B, Dundas SR, Lawrie LC, Telfer C, Murray GI. Characterization and over-expression of chaperonin t-complex proteins in colorectal cancer. J Pathol. 2006;210(3):351–7.
Nibbe RK, Markowitz S, Myeroff L, Ewing R, Chance MR. Discovery and scoring of protein interaction subnetworks discriminative of late stage human colon cancer. Mol Cell Proteomics. 2009;8(4):827–45.
Collins C, Volik S, Kowbel D, Ginzinger D, Ylstra B, Cloutier T, Hawkins T, Predki P, Martin C, Wernick M, Kuo WL, Alberts A, Gray JW. Comprehensive genome sequence analysis of a breast cancer amplicon. Genome Res. 2001;11(6):1034–42.
Miyoshi N, Ishii H, Mimori K, Nishida N, Tokuoka M, Akita H, Sekimoto M, Doki Y, Mori M. Abnormal expression of PFDN4 in colorectal cancer: a novel marker for prognosis. Ann Surg Oncol. 2010;17(11):3030–6.
Vainberg IE, Lewis SA, Rommelaere H, Ampe C, Vandekerckhove J, Klein HL, Cowan NJ. Prefoldin, a chaperone that delivers unfolded proteins to cytosolic chaperonin. Cell. 1998;93(5):863–73.
Miyazawa M, Tashiro E, Kitaura H, Maita H, Suto H, Iguchi-Ariga SM, Ariga H. Prefoldin subunits are protected from ubiquitin-proteasome system-mediated degradation by forming complex with other constituent subunits. J Biol Chem. 2011;286(22):19191–203.
Zako T, Iizuka R, Okochi M, Nomura T, Ueno T, Tadakuma H, Yohda M, Funatsu T. Facilitated release of substrate protein from prefoldin by chaperonin. FEBS Lett. 2005;579(17):3718–24.
Lundin VF, Leroux MR, Stirling PC. Quality control of cytoskeletal proteins and human disease. Trends Biochem Sci. 2010;35(5):288–97.
Geissler S, Siegers K, Schiebel E. A novel protein complex promoting formation of functional alpha- and gamma-tubulin. EMBO J. 1998;17(4):952–66.
Lundin VF, Srayko M, Hyman AA, Leroux MR. Efficient chaperone-mediated tubulin biogenesis is essential for cell division and cell migration in C. elegans. Dev Biol. 2008;313(1):320–34.
Grantham J, Brackley KI, Willison KR. Substantial CCT activity is required for cell cycle progression and cytoskeletal organization in mammalian cells. Exp Cell Res. 2006;312(12):2309–24.
Cao S, Carlesso G, Osipovich AB, Llanes J, Lin Q, Hoek KL, Khan WN, Ruley HE. Subunit 1 of the prefoldin chaperone complex is required for lymphocyte development and function. J Immunol. 2008;181(1):476–84.
O’Neill GM. The coordination between actin filaments and adhesion in mesenchymal migration. Cell Adhes Migr. 2009;3(4):355–7.
Yamaguchi H, Condeelis J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim Biophys Acta. 2007;1773(5):642–52.
Huang HC, Shi J, Orth JD, Mitchison TJ. Evidence that mitotic exit is a better cancer therapeutic target than spindle assembly. Cancer Cell. 2009;16(4):347–58.
Acknowledgments
This work was supported by the National Natural Science Foundation (Grant No. 81201625); Basic Research Project of Shanghai Municipal Science and Technology Commission (Grant No. 13JC1404100); National High Technology Research and Development Program 863 (Grant No. 2012AA021103).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Puxiongzhi Wang and Jingkun Zhao have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, P., Zhao, J., Yang, X. et al. PFDN1, an indicator for colorectal cancer prognosis, enhances tumor cell proliferation and motility through cytoskeletal reorganization. Med Oncol 32, 264 (2015). https://doi.org/10.1007/s12032-015-0710-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-015-0710-z