Abstract
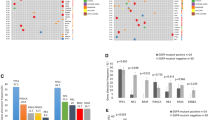
The relationship between epidermal growth factor receptor (EGFR) mutation status and EGFR-tyrosine kinase inhibitors (TKI) efficacy in non-small cell lung cancer (NSCLC) patients has been well established. However, there is no available standard to define the optimal testing method and specimen type required for the detection of EGFR mutations. In this study, we compare results of ADx-amplification refractory mutation system (ARMS) and direct sequencing for the detection of EGFR mutation and prediction of EGFR-TKI efficacy for surgery and biopsy tumor tissues in 158 NSCLC patients. For 71 surgery samples, there were 13 and 17 positive samples detected by direct sequencing and ARMS, respectively. For 87 biopsy samples, direct sequencing and ADx-ARMS found 15 and 32 positive samples, respectively. For surgery samples, sensitivity of direct sequencing and ARMS was 72.2 % (13/18) and 94.4 % (17/18), respectively. For the biopsy samples, sensitivity of direct sequencing and ARMS was 44.1 % (15/34) and 94.1 % (32/34), respectively. For the biopsy and surgery samples, the ORRs for EGFR positive and negative patients detected by direct sequencing were 46.1 versus 16.7 and 66.7 versus 1.1 %, respectively. For ADx-ARMS, the ORR for EGFR positive patients was significantly higher than for negative patients (55.6 vs. 5.6 %). The median progression-free survival time of patients with EGFR wild type detected by direct sequencing (4.2 months) was significantly longer than that of patients with wild type detected by ARMS (1.7 months). ARMS has a higher sensitivity and specificity than direct sequencing for EGFR detection of mutation in both surgical and biopsy samples, and the results from ARMS are more consistent with the efficacy of EGFR-TKIs treatment.



Similar content being viewed by others
References
Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300.
Govindan R, Page N, Morgensztern D, Read W, Tierney R, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24:4539–44.
Sweeney CJ, Zhu J, Sandler AB, Schiller J, Belani CP, et al. Outcome of patients with a performance status of 2 in Eastern Cooperative Oncology Group Study E1594: a phase II trial in patients with metastatic non small cell lung carcinoma. Cancer. 2001;92:2639–47.
Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–8.
Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500.
Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57.
Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–8.
Zhou C, Wu YL, Chen G, Feng J, Liu XQ, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–42.
Han JY, Park K, Kim SW, Lee DH, Kim HY, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30:1122–8.
Kim ES, Hirsh V, Mok T, Socinski MA, Gervais R, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008;372:1809–18.
Zhang L, Ma S, Song X, Han B, Cheng Y, et al. Gefitinib versus placebo as maintenance therapy in patients with locally advanced or metastatic non-small-cell lung cancer (INFORM; C-TONG 0804): a multicentre, double-blind randomised phase 3 trial. Lancet Oncol. 2012;13:466–75.
Kosaka T, Yatabe Y, Endoh H, Kuwano H, Takahashi T, et al. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res. 2004;64:8919–23.
Zhou Q, Zhang XC, Chen ZH, Yin XL, Yang JJ, et al. Relative abundance of EGFR mutations predicts benefit from gefitinib treatment for advanced non-small-cell lung cancer. J Clin Oncol. 2011;29:3316–21.
Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol. 2005;40:90–7.
Zhang X, Zhao Y, Wang M, Yap WS, Chang AY. Detection and comparison of epidermal growth factor receptor mutations in cells and fluid of malignant pleural effusion in non-small cell lung cancer. Lung Cancer. 2008;60:175–82.
Bai H, Mao L, Wang HS, Zhao J, Yang L, et al. Epidermal growth factor receptor mutations in plasma DNA samples predict tumor response in Chinese patients with stages IIIB to IV non-small-cell lung cancer. J Clin Oncol. 2009;27:2653–9.
Santis G, Angell R, Nickless G, Quinn A, Herbert A, et al. Screening for EGFR and KRAS mutations in endobronchial ultrasound derived transbronchial needle aspirates in non-small cell lung cancer using COLD-PCR. PLoS ONE. 2011;6:e25191.
Douillard JY, Shepherd FA, Hirsh V, Mok T, Socinski MA, et al. Molecular predictors of outcome with gefitinib and docetaxel in previously treated non-small-cell lung cancer: data from the randomized phase III INTEREST trial. J Clin Oncol. 2010;28:744–52.
Wu YL, Zhong WZ, Li LY, Zhang XT, Zhang L, et al. Epidermal growth factor receptor mutations and their correlation with gefitinib therapy in patients with non-small cell lung cancer: a meta-analysis based on updated individual patient data from six medical centers in mainland China. J Thorac Oncol. 2007;2:430–9.
Zhang GC, Lin JY, Wang Z, Zhou Q, Xu CR, et al. Epidermal growth factor receptor double activating mutations involving both exons 19 and 21 exist in Chinese non-small cell lung cancer patients. Clin Oncol. 2007;19:499–506.
Masago K, Fujita S, Kim YH, Ichikawa M, Hatachi Y, et al. Epidermal growth factor receptor (EGFR) double-activating somatic mutations in exons 19 and 21 in Japanese non-small cell lung cancer patients. Cancer Genet Cytogenet. 2009;195:179–82.
Li J, Wang L, Mamon H, Kulke MH, Berbeco R, Makrigiorgos GM. Replacing PCR with COLD-PCR enriches variant DNA sequences and redefines the sensitivity of genetic testing. Nat Med. 2008;14:579–84.
Ellison G, Donald E, McWalter G, Knight L, Fletcher L, et al. A comparison of ARMS and DNA sequencing for mutation analysis in clinical biopsy samples. J Exp Clin Cancer Res. 2010;29:132.
Acknowledgments
The authors thank the Bureau of Science and Technology, Guangxi Zhuang Autonomous Zone, China, Grant number: 201017.
Conflict of interest
No potential conflicts of interest were disclosed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shaozhang, Z., Ming, Z., Haiyan, P. et al. Comparison of ARMS and direct sequencing for detection of EGFR mutation and prediction of EGFR-TKI efficacy between surgery and biopsy tumor tissues in NSCLC patients. Med Oncol 31, 926 (2014). https://doi.org/10.1007/s12032-014-0926-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0926-3