Abstract
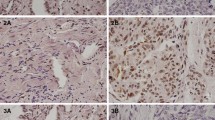
E-cadherin (E-cad) is widely expressed in epithelial cells and acts as a pivotal tumor suppressor. The promoter methylation of E-cad has been reported to closely relate to its downregulation in many kinds of cancers. E-cad expression and methylation status were detected by immunohistochemistry (IHC) and methylation-specific polymerase chain reaction (MS-PCR) in 50 ovarian cancer tissues. 5-Aza-2′-deoxycytidine (5-Aza-dC) was used to demethylate E-cad in SKOV3 and ES2 ovarian cancer cell lines, of which the effect was verified by Western blot and MS-PCR. Then MTT and transwell experiments were conducted to detect the capacity of cell proliferation and migration for these cells. Downregulation of E-cad expression was observed in 60 % of ovarian cancer tissues (30/50) by IHC, whereas MS-PCR result indicated that E-cad was methylated in 64 % of (32/50) ovarian cancer specimens. And E-cad expression was significantly correlated with E-cad methylation (P = 0.004). 5-Aza-dC was used to process SKOV3 and ES2 ovarian cancer cell lines. By MTT experiment, we found that the proliferation of 5-Aza-dC-treated SKOV3 and ES2 was significantly suppressed by 28.0 % (P < 0.05) and 32.3 % (P < 0.05). By transwell experiment, the motility of SKOV3 and ES2 was found to be significantly suppressed by 38.2 and 27.4 % (P < 0.05), respectively, after treated with 5-Aza-dC. E-cad methylation is one of the main reasons for the expression reduction in ovarian cancer. 5-Aza-dC treatment could significantly restore the expression of E-cad and suppress growth and invasion of SKOV3 and ES2 cells. These results suggest E-cad methylation may be a promising target for ovarian cancer therapy.







Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30.
Engholm G, Ferlay J, Christensen N, et al. NORDCAN—a Nordic tool for cancer information, planning, quality control and research. Acta Oncol. 2010;49(5):725–36.
Bhaqat R, Premalata CS, Shilpa V, et al. Altered expression of β-catenin, E-cadherin, and E-cadherin promoter methylation in epithelial ovarian carcinoma. Tumour Biol. 2013;34(4):2459–68.
Moselhy SS, Kumosani TA, Kamal IH, et al. Hypermethylation of P15, P16, and E-cadherin genes in ovarian cancer. Toxicol Ind Health. 2013. doi:10.1177/0748233713484657.
Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3(6):415–28.
Kim YI, Giuliano A, Hatch KD, et al. Global DNA hypomethylation increases progressively in cervical dysplasia and carcinoma. Cancer. 1994;74(3):893–9.
Bedford MT, van Helden PD. Hypomethylation of DNA in pathological conditions of the human prostate. Cancer Res. 1987;47(20):5274–6.
Houshdaran S, Hawley S, Palmer C, et al. DNA methylation profiles of ovarian epithelial carcinoma tumors and cell lines. PLoS ONE. 2010;5(2):e9359.
Barton CA, Hacker NF, Clark SJ, O’Brien PM. DNA methylation changes in ovarian cancer: implications for early diagnosis, prognosis and treatment. Gynecol Oncol. 2008;109(1):129–39.
Mateen S, Raina K, Agarwal C, Chan D, Agarwal R. Silibinin synergizes with histone deacetylase and DNA methyltransferase inhibitors in upregulating E-cadherin expression together with inhibition of migration and invasion of human non-small cell lung cancer cells. J Pharmacol Exp Ther. 2013;345(2):206–14.
Nakagawa H, Hikiba Y, Hirata Y, et al. Loss of liver E-cadherin induces sclerosing cholangitis and promotes carcinogenesis. Proc Natl Acad Sci USA. 2014;111(3):1090–5.
Wei L, Song XR, Sun JJ, Xie L, Wang XW, Lu LY. Effects of lysyl oxidase down-regulation on invasion, migration and E-cadherin protein expression of hypoxic lung cancer cells. Zhonghua Yi Xue Za Zhi. 2012;92(42):3004–7.
Puisieux A. Role of epithelial-mesenchymal transition in tumor progression. Bull Acad Natl Med. 2009;193(9):2017–32; discussion 2032–4.
Brecelj E, Frkovic Grazio S, Auersperg M, Bracko M. Prognostic value of E-cadherin expression in thyroid follicular carcinoma. Eur J Surg Oncol. 2005;31(5):544–8.
Altundag K, Altundag O, Akyurek S, Karakaya E, Turen S. Inactivation of E-cadherin and less sensitivity of lobular breast carcinoma cells to chemotherapy. Breast. 2006;15(3):300.
Cisco RM, Ford JM, Norton JA. Hereditary diffuse gastric cancer: implications of genetic testing for screening and prophylactic surgery. Cancer. 2008;113(7 Suppl):1850–6.
Yoshida J, Horiuchi A, Kikuchi N, et al. Changes in the expression of E-cadherin repressors, Snail, Slug, SIP1, and Twist, in the development and progression of ovarian carcinoma: the important role of Snail in ovarian tumorigenesis and progression. Med Mol Morphol. 2009;42(2):82–91.
Humes HD. Translational medicine and the National Institutes of Health road map: steep grades and tortuous curves. J Lab Clin Med. 2005;146(2):51–4.
Sakai T, Toguchida J, Ohtani N, Yandell DW, Rapaport JM, Dryja TP. Allele-specific hypermethylation of the retinoblastoma tumor-suppressor gene. Am J Hum Genet. 1991;48(5):880–8.
Roman-Gomez J, Castillejo JA, Jimenez A, et al. 5′ CpG island hypermethylation is associated with transcriptional silencing of the p21(CIP1/WAF1/SDI1) gene and confers poor prognosis in acute lymphoblastic leukemia. Blood. 2002;99(7):2291–6.
Gunda V, Cogdill AP, Bernasconi MJ, Wargo JA, Parangi S. Potential role of 5-aza-2′-deoxycytidine induced MAGE-A4 expression in immunotherapy for anaplastic thyroid cancer. Surgery. 2013;154(6):1456–62; discussion 1462.
Reed MD, Tellez CS, Grimes MJ, et al. Aerosolised 5-azacytidine suppresses tumour growth and reprogrammes the epigenome in an orthotopic lung cancer model. Br J Cancer. 2013;109(7):1775–81.
Piekarz RL, Bates SE. Epigenetic modifiers: basic understanding and clinical development. Clin Cancer Res. 2009;15(12):3918–26.
Mostovich LA, Prudnikova TY, Kondratov AG, et al. Integrin alpha9 (ITGA9) expression and epigenetic silencing in human breast tumors. Cell Adh Migr. 2011;5(5):395–401.
Sato N, Maehara N, Su GH, Goggins M. Effects of 5-aza-2′-deoxycytidine on matrix metalloproteinase expression and pancreatic cancer cell invasiveness. J Natl Cancer Inst. 2003;95(4):327–30.
Wang X, Wang H, Jiang N, Lu W, Zhang XF, Fang JY. Effect of inhibition of MEK pathway on 5-aza-deoxycytidine-suppressed pancreatic cancer cell proliferation. Genet Mol Res. 2013;12(4):5560–73.
Yan H, Yu N, Tong J. Effects of 5-Aza-2′-deoxycytidine on the methylation state and function of the WWOX gene in the HO-8910 ovarian cancer cell line. Oncol Lett. 2013;6(3):845–9.
Acknowledgments
This study was supported by Science and Technology Planning Project of Guangdong Province, China (2009B030801317), Medical Scientific Research Foundation of Guangdong Province, China (B2011229), and Youth Foundation of Cancer Hospital of Shantou University Medical College, China [(2009) 5].
Ethical standard
This study was approved by the medical ethics committee of Cancer Hospital of Shantou University Medical College. And written informed consent was obtained from all the patients.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xiao Wu and Yi-xuan Zhuang have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wu, X., Zhuang, Yx., Hong, Cq. et al. Clinical importance and therapeutic implication of E-cadherin gene methylation in human ovarian cancer. Med Oncol 31, 100 (2014). https://doi.org/10.1007/s12032-014-0100-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0100-y