Abstract
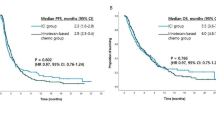
This study aimed to evaluate the efficacy and tolerability of S-1 (Tegafur, Gimeracil, and Oteracil Potassium Capsules) plus CIK (Cytokine-induced killer cells) in patients with advanced pancreatic cancer who had previously received gemcitabine-based therapy. In this prospective study, fifty-eight patients were randomly divided into two groups. One group (CT group) was given S-1 alone, and the other group (immuno-CT group) was given S-1 plus CIK. S-1 was administered orally twice a day at 80 mg/m2/day on days 1–21 of a 28-day cycle till disease progression or unacceptable toxicity occurred. CIK was given for one cycle of 28 days. The disease control rate for S-1 and CIK was 40.0 and 53.6 %, respectively (p = 0.621). The serum CA19-9 level decreased for more than 25 % was significantly different (33.3 and 60.7 % in CT group and immuno-CT group, respectively, p = 0.037). The median time to progression was 2.5 (95 % CI 2.3–2.8) and 2.9 (95 % CI 2.6–3.2) months (p = 0.037) for CT group and immuno-CT group, respectively. The median overall survival was 6.1 (95 % CI 5.7–6.5) and 6.6 (95 % CI 6.1–7.1) months (p = 0.09) for CT group and immuno-CT group, respectively. The difference in hematological toxicity, including leukocytopenia, anemia, and neutropenia, was insignificant between the two groups. In contrast, the differences in non-hematological toxicity, fatigue, and non-infective fever were significantly different between the two groups (p < 0.05). The S-1 plus CIK regimen was well tolerated in a second-line setting in patients with gemcitabine-refractory and advanced pancreatic cancer.


Similar content being viewed by others
References
De Moor V, Arvanitakis M, Nagy N, et al. Intraductal papillary mucinous neoplasms of the pancreas: clinicopathological features and long term outcome related to histopathological group. Hepatogastroenterology. 2012;59:565–9.
Burris HA, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–13.
Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8(1):59–73. doi:10.1038/nri2216.
Gabrilovich DI. Combination of chemotherapy and immunotherapy for cancer: a paradigm revisited. Lancet Oncol. 2007;8(1):2–3.
Smith BD, Kasamon YL, Kowalski J, et al. K562/GMCSF immunotherapy reduces tumor burden in chronic myeloid leukemia patients with residual disease on imatinib mesylate. Clin Cancer Res. 2010;16(1):338–47.
Shirasaka T, Shimamato Y, Ohshimo H, Yamaguchi M, Kato T, Yonekura K, Fukushima M. Development of a novel form of an oral 5-Xuorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-Xuorouracil by two biochemical modulators. Anticancer Drugs. 1996;7:548–57.
Nakai Y, Isayama H, Sasaki T, et al. Impact of S-1 in patients with gemcitabine-refractory pancreatic cancer in Japan. Jpn J Clin Oncol. 2010;40:774–80.
Alsamarai S, Zergebel C, Zhang J, Furuie T, Urrea PD, Saif MW. Long term survival on S-1 monotherapy in a patient with recurrent stage IV pancreatic cancer. JOP. 2008;9:185–91.
Morizane C, Okusaka T, Furuse J, et al. A phase II study of S-1 in gemcitabine-refractory metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2009;63:313–9.
Todaka A, Fukutomi A, Boku N, et al. S-1 monotherapy as second-line treatment for advanced pancreatic cancer after gemcitabine failure. Jpn J Clin Oncol. 2010;40:567–72.
Morizane C, Okusaka T, Furuse J, Ishii H, Ueno H, Ikeda M, Nakachi K, Najima M, Ogura T, Suzuki E. A phase II study of S-1 in gemcitabine-refractory metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2008;63:313–9.
Sudo K, Yamaguchi T, Nakamura K, Denda T, Hara T, Ishihara T, Yokosuka O. Phase II study of S-1 in patients with gemcitabine-resistant advanced pancreatic cancer. Cancer Chemother Pharmacol. 2010; Epub ahead of print.
Baxevanis CN, Gritzapis AD, Tsitsilonis OE, Katsoulas HL, Papamichail M. HER-2/neu-derived peptide epitopes are also recognized by cytotoxic CD3(+)CD56(+) (natural killer T) lymphocytes[J]. Int J Cancer. 2002;98:864–72.
Pancreatic carcinoma-specific immunotherapy using synthesised alpha-galactosyl epitope-activated immune responders: findings from a pilot study.
Okamoto M, Kasetani H, Kaji R, et al. cis-Diamminedichloroplatinum and 5-fluorouracil are potent inducers of the cytokines and natural killer cell activity in vivo and in vitro. Cancer Immunol Immunother. 1998; 47:233Y239.
Okamoto M, Ohe G, Oshikawa T, et al. Induction of cytokines and killer cell activities by cisplatin and 5-fluorouracil in head and neck cancer patients. Anticancer Drugs. 2000; 11:165Y173.
Nakachi K, Furuse J, Ishii H, Suzuki E, Yoshino M. Prognostic factors in patients with gemcitabine-refractory pancreatic cancer. Jpn J Clin Oncol. 2007;37:114–20.
Oettle H, Pelzer U, Stieler J, et al. Oxaliplatin/folinic acid/5- fluorouracil [24 h] (OFF) plus best supportive care versus best supportive care alone (BSC) in second-line therapy of gemcitabine-refractory advanced pancreatic cancer (CONKO 003). J Clin Oncol. 2005;23:4031.
Kim HJ, Yun J, Kim HJ, et al. Phase II study of palliative S-1 in combination with cisplatin as second-line chemotherapy for gemcitabine-refractory pancreatic cancer patients. Oncol Lett. 2012;3(6):1314–8.
Morizane C, Okusaka T, Furuse J, et al. A phase II study of S-1 in gemcitabine-refractory metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2009;63(2):313–9.
Kim MK, Lee KH, Jang BI, et al. S-1 and gemcitabine as an outpatient-based regimen in patients with advanced or metastatic pancreatic cancer. Jpn J Clin Oncol. 2009;39(1):49–53.
Bauer C, Dauer M, Saraj S, et al. Dendritic cell-based vaccination of patients with advanced pancreatic carcinoma: results of a pilot study. Cancer Immunol Immunother. 2011;60(8):1097–107.
Wong D, Ko AH, Hwang J, et al. Serum CA19-9 decline compared to radiographic response as a surrogate for clinical. Pancreas. 2008;37(3):269–2674.
Maisey NR, Norman AR, Hill A, et al. CA19-9 as a prognostic factor in inoperable pancreatic cancer: the implication for clinical trials. Br J Cancer. 2005;93(7):740–3.
Shi SB, Wang M, Niu ZX, et al. Phase II trial of capecitabine combined with thalidomide in second-line treatment of advanced pancreatic cancer. Pancreatology. 2012;12(6):475–9.
Todaka A, Fukutomi A, Boku N, et al. S-1 Monotherapy as second-line treatment for advanced pancreatic cancer after gemcitabine failure. Jpn J Clin Oncol. 2010;40(6):567–72.
Zhong R, Teng J, Han B, et al. Dendritic cells combining with cytokine-induced killer cells synergize chemotherapy in patients with late-stage non-small cell lung cancer. Cancer Immunol Immunother. 2011;60(10):1497–502.
Ma Y, Zhang Z, Tang L, et al. Cytokine-induced killer cells in the treatment of patients with solid carcinomas: a systematic review and pooled analysis. Cytotherapy. 2012;14(4):483–93.
Conflict of interest
No conflicts of interest exist for any author of this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Meng Wang and Sheng-bin Shi have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, M., Shi, Sb., Qi, Jl. et al. S-1 plus CIK as second-line treatment for advanced pancreatic cancer. Med Oncol 30, 747 (2013). https://doi.org/10.1007/s12032-013-0747-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-013-0747-9