Abstract
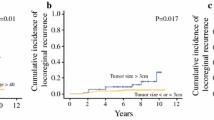
Breast cancers with 10 or more positive lymph nodes at the time of diagnosis are staged as pathological N3a (pN3a) and they have poor prognosis. Recent studies showed five-year disease-free survival (DFS) and overall survival (OS) rates of N3a disease as 43–66 and 58–81 %, respectively. We herein present outcomes of our patients with stage pN3a breast cancer. Among 2,578 patients diagnosed with invasive breast carcinoma at Hacettepe University Hospital between 2002 and 2012, 218 patients (8.4 %) had pN3a disease and were included and analyzed retrospectively in this study. Patients with internal mammary, infraclavicular, and supraclavicular node metastasis or distant metastasis at initial diagnosis were excluded. Demographic features, tumor characteristics, treatment regimens, and patient outcomes in terms of DFS and OS were analyzed. Lymph node ratio was defined as the ratio of positive to total removed lymph nodes. The median age was 49. Most common histological subtype was ductal carcinoma (82.1 %). About 82.6 % of patients had stage T2/T3 cancers and 47.7 % (104) had grade III cancers. Estrogen and progesterone receptors were positive in 133 (61 %) and 121 (55.5 %) patients, respectively. HER2 status was known for 213 patients and was positive in 87 (39.9 %) patients. A total of 27 (12.6 %) patients had triple-negative tumors. Lymphovascular invasion, extracapsular extension, and perineural invasion were present in 106 (48.6 %), 105 (48.2 %), 20 (9.2 %) cases, respectively. A total of 18 patients (8.3 %) received neoadjuvant and 200 patients (91.7 %) received adjuvant chemotherapy, mostly with anthracycline- (95 %) and taxane (60 %)-containing regimens. A total of 210 patients (96.3 %) received radiotherapy. Median follow-up was 39.5 months. A total of 96 patients relapsed on follow-up and 64 patients died. Nineteen of the relapses were locoregional and 77 were distant relapses. The 5-year DFS rate was 46.2 % and the OS rate was 69.8 %. In multivariate Cox regression analysis, grade III disease (HR 1.899, 95 % CI 1.196–3.017, P = 0.007), perineural invasion (HR 2.519, 95 % CI 1.341–4.731, P = 0.004), and lymph node ratio (≥0.9 vs. <0.9) (HR 2.290, 95 % CI 1.368–3.835, P = 0.002) were significantly associated with DFS, and grade III disease (HR 2.679, 95 % CI 1.500–4.782, P = 0.001) and lymph node ratio (≥0.9 vs. <0.9) (HR 2.182, 95 % CI 1.211–3.932, P = 0.009) were significantly associated with OS. Patients with pN3a disease in our cohort have comparable survival rates with other reports in the literature. Within this high risk group of patients, those with grade III disease, perineural invasion, and lymph node ratio ≥0.9 seem to confer poorer prognosis.


Similar content being viewed by others
References
Hortobagyi GN, de la Garza Salazar J, Pritchard K, et al. The global breast cancer burden: variations in epidemiology and survival. Clin Breast Cancer. 2005;6:391–401.
Glass AG, Lacey JV Jr, Carreon JD, et al. Breast cancer incidence, 1980–2006: combined roles of menopausal hormone therapy, screening mammography, and estrogen receptor status. J Natl Cancer Inst. 2007;99:1152–61.
Newman LA. Epidemiology of locally advanced breast cancer. Semin Radiat Oncol. 2009;19:195–203.
Cianfrocca M, Goldstein LC. Prognostic and predictive factors in early-stage breast cancer. Oncologist. 2004;9:606–16.
Singletary SE, Allred C, Ashley P, et al. Staging system for breast cancer: revisions for the 6th edition of the AJCC cancer staging manual. SurgClin North Am. 2003;83:803–19.
Basaran G, Devrim C, Caglar HB, Gulluoglu B, Kaya H, Seber S, Korkmaz T, Telli F, Kocak M, Dane F, Yumuk FP, Turhal SN. Clinical outcome of breast cancer patients with N3a (≥10 positive lymph nodes) disease: has it changed over years? Med Oncol. 2011;28:726–32.
Lee JS, Kim SI, Choi SY, Park HS, Lee JS, Park S, Koo J, Park BW, Lee KS. Factors influencing the outcome of breast cancer patients with 10 or more metastasized axillary lymph nodes. Int J Clin Oncol. 2011;16:473–81.
van de Vijver MJ, He YD, van’t Veer LJ, et al. A gene expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009.
Montero AJ, Rouzier R, Lluch A, et al. The natural history of breast carcinoma in patients with [or = 10 metastatic axillary lymph nodes before and after the advent of adjuvant therapy: a multiinstitutional retrospective study. Cancer. 2005;104:229–35.
Duraker N, Caynak ZC, Bati B. Is there any prognostically different subgroup among patients with stage IIIC (any TN3M0) breast carcinoma? Ann SurgOncol. 2008;15:430–7.
Buzdar AU, et al. Clinical course of patients with breast cancer with ten or more positive nodes who were treated with doxorubicin-b containing adjuvant therapy. Cancer. 1992;69(2):448–52.
Walker MS, et al. The natural history of breast cancer with more than 10 positive nodes. Am J Surg. 1995;169:575–9.
Schmoor C, Sauerbrei W, Bastert G, Bojar H, Schumacher M. German breast cancer study group. Long-term prognosis of breast cancer patients with 10 or more positive lymph nodes treated with CMF. Eur J Cancer. 2001;37:1123–31.
Ito M, Moriya T, Ishida T, et al. Significance of pathological evaluation for lymphatic vessel invasion in invasive breastcancer. Breast Cancer. 2007;14:381–7.
Schoppmann SF, Bayer G, Aumayr K, et al. Prognostic value of lymphangiogenesis and lymphovascular invasion in invasive breast cancer. Ann Surg. 2004;240:306–12.
Woodward WA, Vinh-Hung V, Ueno NT, Cheng YC, Royce M, Tai P, Vlastos G, Wallace AM, Hortobagyi GN, Nieto Y. Prognostic value of nodal ratios in node-positive breast cancer. J Clin Oncol. 2006;24:2910–6.
Schiffman SC, McMasters KM, Scoggins CR, Martin RC, Chagpar AB. Lymph node ratio: a proposed refinement of current axillary staging in breast cancer patients. J Am Coll Surg. 2011;213:45–52.
van der Wal BC, Butzelaar RM, van der Meij S, Boermeester MA. Axillary lymph node ratio and total number of removed lymph nodes: predictors of survival in stage I and II breast cancer. Eur J Surg Oncol. 2002;28:481–9.
Zhu C, Wu XZ. Proposal of new classification for stage III breast cancer on the number and ratio of metastatic lymph nodes. J Surg Oncol. 2012;106:696–702.
Chagpar AB, Camp RL, Rimm DL. Lymph node ratio should be considered for incorporation into staging for breast cancer. Ann Surg Oncol. 2011;18:3143–8.
Vinh-Hung V, Verkooijen HM, Fioretta G, Neyroud-Caspar I, Rapiti E, Vlastos G, Deglise C, Usel M, Lutz JM, Bouchardy C. Lymph node ratio as an alternative to pN staging in node-positive breast cancer. J Clin Oncol. 2009;27:1062–8.
Geara FB, Nasr E, Tucker SL, Charafeddine M, Dabaja B, Eid T, Abbas J, Salem Z, Shamseddine A, Issa P, El Saghir N. Breast cancer patients with 10 or more involved axillary lymph nodes treated by multimodality therapy: influence of clinical presentation on outcome. Int J Radiat Oncol Biol Phys. 2007;68:364–9.
Petrelli F, Borgonovo K, Barni S. The emerging issue of ratio of metastatic to resected lymph nodes in gastrointestinal cancers: an overview of literature. Eur J Surg Oncol. 2011;37:836–47.
Schneider DF, Chen H, Sippel RS. Impact of lymph node ratio on survival in papillary thyroid cancer. Ann Surg Oncol. 2012 Dec 23. [Epub ahead of print].
John BJ, Naik P, Ironside A, Davidson BR, Fusai G, Gillmore R, Watkins J, Rahman SH. Redefining the R1 resection for pancreatic ductal adenocarcinoma: tumour lymph nodal burden and lymph node ratio are the only prognostic factors associated with survival. HPB (Oxford). 2013;. doi:10.1111/hpb.12019.
Qiu C, Dong W, Su B, Liu Q, Du J. The prognostic value of ratio-based lymph node staging in resected non-small-cell lung cancer. J Thorac Oncol. 2013;8:429–35.
Conflict of interest
There is no conflict of interest for all authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koca, E., Kuzan, T.Y., Dizdar, O. et al. Outcomes of locally advanced breast cancer patients with ≥10 positive axillary lymph nodes. Med Oncol 30, 615 (2013). https://doi.org/10.1007/s12032-013-0615-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-013-0615-7