Abstract
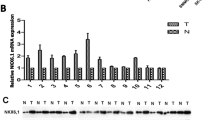
Although the role of Krüppel-like factor 17 (KLF17) in regulating epithelial–mesenchymal transition (EMT) has been explored in breast cancer, its influence on primary hepatocellular carcinoma (HCC) remains unclear. This study aims to investigate the expression status of KLF17 in hepatocellular carcinoma (HCC) and the correlation between KLF17 expression and metastatic potential of HCC. KLF17 expression in HCC and adjacent liver tissues was studied by real-time PCR and Western blot, and the relationship between KLF17 expression and the clinicopathological features of HCC was evaluated in 60 patients. By using RNA interference technique, the correlation of KLF17 expression and metastatic potential was investigated by down-regulating KLF17 expression in HepG2 cells, and the effects of KLF17 down-regulation on cell migration, and invasion were then analyzed. Furthermore, the correlation between KLF17 expression and the surgical outcomes of a cohort of HCC patients was analyzed. Reduced expression of KLF17 is associated with a short survival time in clinical patients (P = 0.034). Low KLF17 expression is related to tumor T stage (P = 0.045), tumor size (P = 0.027), lymph node stage (P = 0.030), M stage (P = 0.048), and portal vein tumor thrombosis significantly in HCC. Reduced expression of KLF17 promoted motility and invasion ability of HepG2 cells and changed the expression of E-cadherin, ZO-1, Snai1, and vimentin (genes are associated with EMT). Overall, these findings suggest a repressing role of KLF17 in tumor invasion and a new prognostic indicator in directing therapy. It deserves further exploration.



Similar content being viewed by others
References
Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6(9):674–87.
Liotta LA. Mechanisms of cancer invasion and metastasis. Important advances in oncology. 1985:28–41. (Epub 1985/01/01).
Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–90.
Suske G, Bruford E, Philipsen S. Mammalian SP/KLF transcription factors: bring in the family. Genomics. 2005;85(5):551–6.
van Vliet J, Crofts LA, Quinlan KG, Czolij R, Perkins AC, Crossley M. Human KLF17 is a new member of the Sp/KLF family of transcription factors. Genomics. 2006;87(4):474–82.
Kim J, Shin S, Subramaniam M, Bruinsma E, Kim TD, Hawse JR, et al. Histone demethylase JARID1B/KDM5B is a corepressor of TIEG1/KLF10. Biochem Biophys Res Commun. 2010;401(3):412–6.
Spittau G, Happel N, Behrendt M, Chao TI, Krieglstein K, Spittau B. Tieg1/Klf10 is upregulated by NGF and attenuates cell cycle progression in the pheochromocytoma cell line PC12. J Neurosci Res. 2010;88(9):2017–25.
Kaczynski J, Cook T, Urrutia R. Sp1- and Kruppel-like transcription factors. Genome Biol. 2003;4(2):206.
Humbert M, Halter V, Shan D, Laedrach J, Leibundgut EO, Baerlocher GM, et al. Deregulated expression of Kruppel-like factors in acute myeloid leukemia. Leukemia Res. 2011;35(7):909–13.
McConnell BB, Yang VW. Mammalian Kruppel-like factors in health and diseases. Physiol Rev. 2010;90(4):1337–81.
Antin PB, Pier M, Sesepasara T, Yatskievych TA, Darnell DK. Embryonic expression of the chicken Kruppel-like (KLF) transcription factor gene family. Dev Dyn. 2010;239(6):1879–87.
X-d Cai, Y-b Zhou, L-x Huang, Q-l Zeng, L-j Zhang, Q-q Wang, et al. Reduced expression of Krüppel-like factor 17 is related to tumor growth and poor prognosis in lung adenocarcinoma. Biochem Biophys Res Comm. 2012;418(1):67–73.
Gumireddy K, Li A, Gimotty PA, Klein-Szanto AJ, Showe LC, Katsaros D, et al. KLF17 is a negative regulator of epithelial-mesenchymal transition and metastasis in breast cancer. Nat Cell Biol. 2009;11(11):1297–304.
Fernandez-Zapico ME, Lomberk GA, Tsuji S, DeMars CJ, Bardsley MR, Lin YH, et al. A functional family-wide screening of SP/KLF proteins identifies a subset of suppressors of KRAS-mediated cell growth. Biochem J. 2011;435(2):529–37.
Vetter D, Cohen-Naftaly M, Villaneuva A, Lee YA, Kocabayoglu P, Hannivoort R, et al. Enhanced hepatocarcinogenesis in mouse models and human HCC by coordinate KLF6 depletion and increased mRNA splicing. Hepatology. 2012. (Epub 2012/04/27).
Tarocchi M, Hannivoort R, Hoshida Y, Lee UE, Vetter D, Narla G, et al. Carcinogen-induced hepatic tumors in KLF6 ± mice recapitulate aggressive human hepatocellular carcinoma associated with p53 pathway deregulation. Hepatology. 2011;54(2):522–31.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–15.
Sleeman JP, Thiery JP. SnapShot: the epithelial-mesenchymal transition. Cell. 2011;145(1):162.
Ishiyama N, Lee SH, Liu S, Li GY, Smith MJ, Reichardt LF, et al. Dynamic and static interactions between p120 catenin and E-cadherin regulate the stability of cell–cell adhesion. Cell. 2010;141(1):117–28.
Patel SD, Ciatto C, Chen CP, Bahna F, Rajebhosale M, Arkus N, et al. Type II cadherin ectodomain structures: implications for classical cadherin specificity. Cell. 2006;124(6):1255–68.
Philippova M, Joshi MB, Kyriakakis E, Pfaff D, Erne P, Resink TJ. A guide and guard: the many faces of T-cadherin. Cell Signal. 2009;21(7):1035–44.
Chan DW, Lee JMF, Chan PCY, Ng IOL. Genetic and epigenetic inactivation of T-cadherin in human hepatocellular carcinoma cells. Int J Cancer. 2008;123(5):1043–52.
Satelli A, Li S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol Life Sci. 2011;68(18):3033–46.
Savagner P. The epithelial–mesenchymal transition (EMT) phenomenon. Ann Oncol. 2010;21(Suppl 7):789–92.
Korpal M, Ell BJ, Buffa FM, Ibrahim T, Blanco MA, Celia-Terrassa T, et al. Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nat Med. 2011;17(9):1101–8.
Ellenrieder V. TGFβ-regulated gene expression by smads and Sp1/KLF-like transcription factors in cancer. Anti cancer Res. 2008;28(3):1531–9.
Acknowledgments
We thank Professor Zhiyong Huang of the Department of Surgery (Tongji Hospital of Tongji Medical College of Huazhong University of Science and Technology, China) for the providing the samples of patients with HCC.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service, and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled, “Down-regulated KLF17 expression is associated with tumor invasion and poor prognosis in hepatocellular carcinoma.”
Author information
Authors and Affiliations
Corresponding authors
Additional information
Fu-Yao Liu and Yue-Ling Deng contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, FY., Deng, YL., Li, Y. et al. Down-regulated KLF17 expression is associated with tumor invasion and poor prognosis in hepatocellular carcinoma. Med Oncol 30, 425 (2013). https://doi.org/10.1007/s12032-012-0425-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-012-0425-3