Abstract
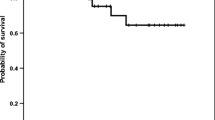
The soluble forms of Fas and its ligand (sFas and sFasL) correlate with disease progression in various malignancies. We compared serum levels of sFas and sFasL in children with acute lymphoblastic leukemia and healthy children to determine the prognostic significance of these molecules. Serum levels of sFas and sFasL were measured with an enzyme-linked immunosorbent assay in 48 patients with newly diagnosed childhood acute lymphoblastic leukemia and 38 healthy children. Cut-off values of sFas and sFasL levels were based on their levels in controls. Clinical and laboratory characteristics were recorded on admission. The mean serum concentration of sFas was 243 ± 40 pg/mL in patients and 238 ± 29 pg/mL in controls. Serum levels of sFasL were 4.33 ± 0.25 ng/mL in patients and 4.27 ± 0.11 ng/mL in controls. Neither difference was significant. Based on the cut-off value, 12.5% of the patients were positive for sFas, and 16.6% were positive for sFasL. Survival was significantly longer in sFasL-positive patients (394 ± 69.6 vs. 254 ± 24.3 days) and the duration of complete remission was also longer (380 ± 65.0 vs. 246 ± 26.0 days) than in sFasL-negative patients (P < 0.02), indicating the important role of this molecule in the response to therapy. Higher sFas levels were associated with hepatosplenomegaly (P < 0.047). In conclusion, sFasL positivity was associated with a favorable outcome in ALL patients.


Similar content being viewed by others
References
Onciu M. Acute lymphoblastic leukemia. Hematol Oncol Clin North Am. 2009;23:655–74.
Ribera JM, Oriol A. Acute lymphoblastic leukemia in adolescents and young adults. Hematol Oncol Clin North Am. 2009;23:1033–42.
Whitlock JA, Gaynon PS. Acute lymphoblastic leukemia in children. In: Greer JP, Foerster J, Lukens JN, editors. Wintrobe’s Clinical Hematology, 11th ed. 11th ed. Philadelphia: Lippincott Williams & Wilkins, Lippincott; 2003. p. 4298–354.
Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008;371:1030–43.
Pieters R. Acute lymphoblastic leukemia in children and adolescents: chance of cure now higher than 80%. Ned Tijdschr Geneeskd. 2010;154:A1577.
Rowe JM. Prognostic factors in adult acute lymphoblastic leukaemia. Br J Haematol. 2010;150:389–405.
Iannolo G, Conticello C, Memeo L, et al. Apoptosis in normal and cancer stem cells. Crit Rev Oncol Hematol. 2008;66:42–51.
Zimmermann KC, Bonzon C, Green DR. The machinery of programmed cell death. Pharmacol Ther. 2001;92:57–70.
Guicciardi ME, Gores GJ. Life and death by death receptors. Faseb J. 2009;23:1625–37.
Lavrik I, Golks A, Krammer PH. Death receptor signaling. J Cell Sci. 2005;11:265–7.
Peter ME, Krammer PH. The CD95 (APO–1/Fas) disc and beyond. Cell Death Differ. 2003;10:26–35.
Krammer PH, Arnold R, Lavrik IN. Life and death in peripheral T cells. Nat Rev Immunol. 2007;7:532–42.
Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis. 2002;21:485–95.
Igney FH, Krammer PH. Death and anti–death, tumor resistance to apoptosis. Nature Rev Cancer. 2002;2:277–88.
Reichmann E. The biological role of the Fas/FasL system during tumor formation and progression. Semin Cancer Biol. 2002;12:309–15.
Yang J, Epling-Burnette PK, Painter JS, et al. Antigen activation and impaired Fas–induced death–inducing signaling complex formation in T–large–granular lymphocyte leukemia. Blood. 2008;111:1610–6.
Yolcu ES, Ash S, Kaminitz A, Sagiv Y, Askenasy N, Yarkoni S. Apoptosis as a mechanism of T–regulatory cell homeostasis and suppression apoptosis in T–regulatory cell homeostasis. Immunol Cell Biol. 2008;86:650–8.
Urbaniank KD, Jazwies B, Tomaszewska TB, et al. Expression of Fas receptor and sFasL concentration in acute leukemia. Pol Arch Wewn. 2002;108:873–8.
Liu X, Qi Z, Luo L, Zhang X (1999) Measurement of soluble Fas in patients with hematological malignancy. Hunan Yi Ke Da Xue Xue Bao 24:171–173, 176.
Kapplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Statist Assoc. 1958;53:457–81.
Schimmer AD. Apoptosis in leukemia: from molecular pathways to targeted therapies. Best Pract Res Clin Haematol. 2008;21:5–11.
Jattela M. Multiple cell death pathways are regulators of tumor initiation and progression. Oncogene. 2004;23:2746–56.
Krueger A, Fas SC, Baumann S, et al. The role of CD95 in the regulation of peripheral T-cell apoptosis. Immunol Rev. 2003;193:58–69.
Amirghofran Z, Bahmani M, Azadmehr A, et al. Anticancer effects of various Iranian native medicinal plants on human tumor cell lines. Neoplasma. 2006;53:428–33.
Amirghofran Z, Daneshbod Y, Gholijani N. Bcl–2 in combination to myeloid antigen expression in adult acute lymphoblastic leukemia and prognostic outcome. Oncol Res. 2009;17:447–54.
Li-Weber M, Krammer PH. Function and regulation of the CD95 (APO-1/Fas) ligand in the immune system. Semin Immunol. 2003;15:145–57.
Barnhart BC, Legembre P, Pietras E, et al. CD95 ligand induces motility and invasiveness of apoptosis–resistant tumor cells. EMBO J. 2004;23:3175–85.
Pommier Y, Sordet O, Antony S, et al. Apoptosis defects and chemotherapy resistance. Oncogene. 2004;23:2934–49.
Mata JF, Silveira VS, Mateo EC, et al. Low mRNA expression of the apoptosis–related genes CASP3, CASP8, and Fas is associated with low induction treatment response in childhood acute lymphoblastic leukemia (ALL). Pediatr Blood Cancer. 2010;55:100–7.
Li Q, Tsuruda K, Sugahara KN, et al. Qualitative and quantitative characterization of Fas (CD95) expression and its role in primary human acute leukemia cells. Leuk Res. 2000;24:437–44.
Vries EG, Timmer T, Mulder NH, et al. Modulation of death receptor pathways in oncology. Drugs Today (Barc). 2003;39(Suppl):95–109.
Wuchter C, Karawajew L, Ruppert V, et al. Constitutive expression levels of CD95 and Bcl–2 as well as CD95 function and spontaneous apoptosis in vitro do not predict the response to induction chemotherapy and relapse rate in childhood acute lymphoblastic leukemia. Br J Hematol. 2000;110:154–60.
Min YH, Lee S, Lee JW, et al. Expression of Fas antigen in acute myeloid leukemia is associated with therapeutic response to chemotherapy. Br J Hematol. 1996;93:928–30.
Hentschel N, Krusch M, Kiener PA, et al. Serum levels of sCD137 (4–1BB) ligand are prognostic factors for progression in acute myeloid leukemia but not in non-Hodgkin’s lymphoma. Eur J Haematol. 2006;77:91–101.
Igney FH, Behrens CK, Krammer PH. CD95L mediates tumor counterattack in vitro but induces neutrophil-independent tumor rejection in vivo. Int J Cancer. 2005;113:78–87.
Hohlbaum AM, Moe S, Marshak-Rothstein A. Opposing effects of transmembrane and soluble Fas ligand expression on inflammation and tumor cell survival. J Exp Med. 2000;191:1209–20.
Suda T, Hashimoto H, Tanaka M, et al. Membrane Fas ligand kills human peripheral blood T lymphocytes, and soluble Fas ligand blocks the killing. J Exp Med. 1997;186:2045–50.
Pearl-Yafe M, Yolcu ES, Yaniv I, et al. The dual role of Fas-ligand as an injury effector and defense strategy in diabetes and islet transplantation. Bioessays. 2006;28:211–22.
Abbasova SG, Vysotskii MM, Ovchinnikova LK. Cancer and soluble Fas. Bull Exp Biol Med. 2009;148:638–42.
Osorio LM, Aguilar-Santelises M, De Santiago A, et al. Increased serum levels of soluble Fas in progressive B-CLL. Eur J Haematol. 2001;66:342–6.
Ebeid EN, Khairy A, Amin M, et al. Soluble CD95 (APO–1/Fas) level in infancy and childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23(suppl):8540.
Hazar V, Berber Z, Pestereli E, et al. Clinical importance of circulating and cellular expression levels of Fas and Fas ligand in pediatric patients with lymphoproliferative malignancies. Pediatr Hematol Oncol. 2005;22:247–56.
Courtney PA, Crockard AD, Williamson K, et al. Lymphocyte apoptosis in systemic lupus erythematosus: relationships with Fas expression, serum soluble Fas and disease activity. Lupus. 1999;8:508–13.
Herrmann M, Voll RE, Zoller OM, et al. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41:1241–50.
Acknowledgments
This work was supported by grant no. 4724 from Shiraz University of Medical Sciences. We thank Saied Malek Hoseini and the head nurses of the pediatric oncology ward of Amir Hospital in Shiraz for help with liaising with the patients, K. Shashok (AuthorAID in the Eastern Mediterranean) for improving the use of English in the manuscript, and M. Gholami at the Center for Development of Clinical Research of Nemazee Hospital for research assistance.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fathi, M., Amirghofran, Z. & Shahriari, M. Soluble Fas and Fas ligand and prognosis in children with acute lymphoblastic leukemia. Med Oncol 29, 2046–2052 (2012). https://doi.org/10.1007/s12032-011-9965-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-011-9965-1