Abstract
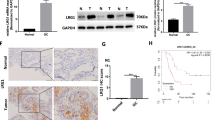
A disintegrin and metalloproteinase-17 (ADAM17, also named as tumor necrosis factor-alpha-converting enzyme) is a member of the ADAM family. Of all ADAMs, the strongest evidence for a role in malignancy exists for ADAM17. Especially, it has been demonstrated that ADAM17 expression was significantly increased in human gastric cancer. The aim of this study was to investigate the association between ADAM17 expression and the clinicopathological features of patients with gastric cancer. The expression of ADAM17 was detected by real-time quantitative RT-PCR in gastric cancer and adjacent non-cancerous tissues. In addition, ADAM17 expression was analyzed by immunohistochemistry in 220 clinicopathologically characterized gastric cancer cases. The expression levels of ADAM17 mRNA and protein in gastric cancer tissues were both significantly higher than those in non-cancerous gastric mucosa. In addition, positive expression of ADAM17 correlated with the degree of tumor differentiation, depth of invasion, lymph node metastases, distant metastases, and TNM stage (all P < 0.05). Furthermore, multivariate analysis suggested that lymph node metastases, distant metastases, TNM stage, and ADAM17 expression were independent prognostic indicators for gastric cancer. Our data suggest for the first time that the increased expression of ADAM17 in gastric cancer is associated significantly with aggressive progression and poor prognosis. ADAM17 may be an important molecular marker for predicting the carcinogenesis, progression, and prognosis of gastric cancer.



Similar content being viewed by others
References
Zhang YZ, Zhang LH, Gao Y, et al. Discovery and validation of prognostic markers in gastric cancer by genome-wide expression profiling. World J Gastroenterol. 2011;17:1710–7.
Ye YW, Dong RZ, Zhou Y, et al. Prognostic analysis of familial gastric cancer in Chinese population. J Surg Oncol. 2011;104:76–82.
Edwards DR, Handsley MH, Pennington CJ. The ADAM metalloproteinases. Mol Aspects Med. 2008;29:258–89.
Murphy G. The ADAMs: signaling scissors in the tumour microenvironment. Nat Rev Cancer. 2008;8:929–41.
Duffy MJ, McKiernan E, O’Donovan N, et al. Role of ADAMs in cancer formation and progression. Clin Cancer Res. 2007;13:2335–43.
Wang YY, Ye ZY, Li L, Zhao ZS, Shao QS, Tao HQ. ADAM 10 is associated with gastric cancer progression and prognosis of patients. J Surg Oncol. 2011;103:116–23.
Xu P, Derynck R. Direct activation of TACE-mediated ectodomain shedding by p38 MAP kinase regulates EGF receptor-dependent cell proliferation. Mol Cell. 2010;37:551–66.
Kenny PA, Bissell MJ. Targeting TACE-dependent EGFR ligand shedding in breast cancer. J Clin Invest. 2007;117:337–45.
Bozkulak EC, Weinmaster G. Selective use of ADAM10 and ADAM17 in activation of Notch1 signaling. Mol Cell Biol. 2009;29:5679–95.
Szalad A, Katakowski M, Zheng X, et al. Transcription factor Sp1 induces ADAM17 and contributes to tumor cell invasiveness under hypoxia. J Exp Clin Cancer Res. 2009;28:129.
Yoshimura T, Tomita T, Dixon MF, et al. ADAMs (a disintegrin and metalloproteinase) messenger RNA expression in Helicobacter pylori-infected, normal, and neoplastic gastric mucosa. J Infect Dis. 2002;185:332–40.
Yasuda H, Hirata S, Inoue K, et al. Involvement of membrane-type bile acid receptor M-BAR/TGR5 in bile acid-induced activation of epidermal growth factor receptor and mitogen-activated protein kinases in gastric carcinoma cells. Biochem Biophys Res Commun. 2007;354:154–9.
Ebi M, Kataoka H, Shimura T, et al. TGFβ induces proHB-EGF shedding and EGFR transactivation through ADAM activation in gastric cancer cells. Biochem Biophys Res Commun. 2010;402:449–54.
Arribas J, Bech-Serra JJ, Santiago-Josefat B. ADAMs, cell migration and cancer. Cancer Met Rev. 2006;25:57–68.
Duffy MJ, McKiernan E, O’Donovan N, et al. The role of ADAMs in disease pathophysiology. Clin Chim Acta. 2009;403:31–6.
Scheller J, Chalaris A, Garbers C, Rose-John S. ADAM17: a molecular switch to control inflammation and tissue regeneration. Trends Immunol. 2011;32:380–7.
Willems SH, Tape CJ, Stanley PL, et al. Thiol isomerases negatively regulate the cellular shedding activity of ADAM17. Biochem J. 2010;428:439–50.
Lin P, Sun X, Feng T, et al. ADAM17 regulates prostate cancer cell proliferation through mediating cell cycle progression by EGFR/PI3K/AKT pathway. 2011 In press.
Stokes A, Joutsa J, Ala-Aho R, et al. Expression profiles and clinical correlations of degradome components in the tumor microenvironment of head and neck squamous cell carcinoma. Clin Cancer Res. 2010;16:2022–35.
Baumgart A, Seidl S, Vlachou P, et al. ADAM17 regulates epidermal growth factor receptor expression through the activation of Notch1 in non-small cell lung cancer. Cancer Res. 2010;70:5368–78.
McGowan PM, Ryan BM, Hill AD, McDermott E, O’Higgins N, Duffy MJ. ADAM-17 expression in breast cancer correlates with variables of tumor progression. Clin Cancer Res. 2007;13:2335–43.
Saftig P, Reiss K. The “A Disintegrin And Metalloproteases” ADAM10 and ADAM17: novel drug targets with therapeutic potential? Eur J Cell Biol. 2011;90:527–35.
Sinnathamby G, Zerfass J, Hafner J, et al. ADAM metallopeptidase domain 17 (ADAM17) is naturally processed through major histocompatibility complex (MHC) class I molecules and is a potential immunotherapeutic target in breast, ovarian and prostate cancers. Clin Exp Immunol. 2011;163:324–32.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Tc., Zhu, Wg., Huang, Md. et al. Prognostic value of ADAM17 in human gastric cancer. Med Oncol 29, 2684–2690 (2012). https://doi.org/10.1007/s12032-011-0125-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-011-0125-4