Abstract
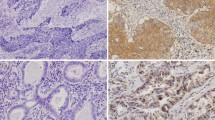
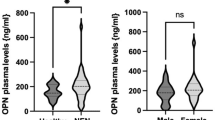
The aim of this study was to evaluate the usefulness of osteopontin (OPN) expression level in plasma and tumor tissues of patients with laryngeal and hypopharyngeal squamous-cell carcinoma for predicting metastasis and survival of this tumor. The OPN expression in tumor tissues was detected by immunohistochemical staining in a tissue microarray of laryngeal and hypopharyngeal carcinomas, and the OPN level in plasma was measured by ELISA. The expression levels of OPN in plasma and tumor tissues were associated with clinicopathological features and survival of laryngeal and hypopharyngeal carcinomas. Results showed that the OPN expression quantitation either in tissues or plasma was significantly correlated with differentiation and lymphatic metastasis of the laryngeal and hypopharyngeal carcinoma. Elevated OPN level of plasma and tissues was significantly associated with poor survival. In conclusion, elevated OPN level in plasma and tumor tissues was significantly associated with metastasis and survival of laryngeal and hypopharyngeal carcinomas. Elevated OPN level in plasma and tumor tissues may become a useful indicator of prognosis for laryngeal and hypopharyngeal cancers.



Similar content being viewed by others
References
Wai PY, Kuo PC. Osteopontin: regulation in tumor metastasis. Cancer Metastasis Rev. 2008;27:103–18.
Weber GF, Ashkar S, Glimcher MJ, Cantor H. Receptor-ligand interaction between CD44 and osteopontin. Science. 1996;271:509–12.
Katagiri YU, Sleeman J, Fujii H, Herrlich P, Hotta H, Tanaka K, Chikuma S, Yagita H, Okumura K, Murakami M, Saiki I, Chambers AF, Uede T. CD44 variants but not CD44S cooperated with betal-containing integrins to permit cell bind to osteopontin indcpend of arginine-glycine-aspartic acid, there by stimulating cell motility and chemotaxis. Cancer Res. 1999;59(1):219–26.
EI Tanani MK, Campbell FC, Kurisetty V, Jin D, McCann M, Rudland PS. The regulation and role of osteopontin in malignant transformation and cancer. Cytokine Growth Factor Rev. 2006;17:463–74.
Jweber GF. The metastasis gene osteopontin: a candidate target for cancer therapy. Biochim Biophys Acta. 2001;1552(2):61–85.
Crosby AH, Edwards SJ, Murray JC, Dixon MJ. Genomic organization of the human osteopontin gene:exclusion of the locus from a causative role in the pathogenesis of dentinogenesis impefecta typeII. Genomics. 1995;27(1):155–60.
Meerovitch K, Bergeron F, Leblond L, Grouix B, Poirier C, Bubenik M, Chan L, Gourdeau H, Bowlin T, Attardo G. A novel RGD antagonist that targets both alphavbeta3 and alpha5beta1 induces apoptosis of angiogenic endothelial cells on type I collagen. Vasc Pharmacol. 2003;40(2):77–89.
Crawford HC, Matrisian LM, Liaw L. Distinct roles of osteopontin in host defense activity and tumor survival during squamous cell carcinoma progression in vivo. Cancer Res. 1998;58:5206–15.
Gillespie MT, Thomas RJ, Pu ZY, Zhou H, Martin TJ, Findlay DM. Calcitonin receptors, bone sialoprotein and osteopontin are expressed in primary breast cancers. Int J Cancer. 1997;73:812–5.
Kim JH, Skates SJ, Uede T, Wong KK, Schorge JO, Feltmate CM, Berkowitz RS, Cramer DW, Mok SC. Osteopontin as a potential diagnostic biomarker for ovarian cancer. J Am Med Assoc. 2002;287:1671–9.
Coppola D, Szabo M, Boulware D. Correlation of osteopontin protein expression and pathological stage across a wide variety of tumor histologies. Clin Cancer Res. 2004;10:184–90.
Shimada Y, Watanabe G, Kawamura J, Soma T, Okabe M, Ito T, Inoue H, Kondo M, Mori Y, Tanaka E, Imamura M. Clinical significance of osteopontin in esophageal squamous cell carcinoma: comparison with common tumor markers. Oncology. 2005;68:285–92.
Fedarko NS, Jain A, Karadag A, Van Eman MR, Fisher LW. Elevated serum bone sialoprotein and osteopontin in colon, breast, prostate, and lung cancer. Clin Cancer Res. 2001;7:4060–6.
Hotte SJ, Winquist EW, Stitt L, Wilson SM, Chambers AF. Plasma osteopontin: associations with survival and metastasis to bone in men with hormone-refractory prostate carcinoma. Cancer. 2002;95(3):506–12.
Lu JG, Sun YN, Wang C, de Jin J, Liu M. Role of the alpha v-integrin subunit in cell proliferation, apoptosis and tumor metastasis of laryngeal and hypopharyngeal squamous cell carcinomas: a clinical and in vitro investigation. Eur Arch Otorhinolaryngol. 2009;266:89–96.
Celetti A, Testa D, Staibano S, Merolla F, Guarino V, Castellone MD, Iovine R, Mansueto G, Somma P, De Rosa G, Galli V, Melillo RM, Santoro M. Overexpression of the cytokine osteopontin identifies aggressive laryngeal squamous cell carcinomas and enhances carcinoma cell proliferation and invasiveness. Clin Cancer Res. 2005;15:8019–27.
Mao L, Hong WK, Papadimitrakopoulou VA. Focus on head and neck cancer. Cancer Cell. 2004;5:311–6.
Liu M, Lawson G, Delos M, Jamart J, Remacle M. Expression of E-Cadherin adhesion molecule in vocal cord carcinoma. Eur Arch Otorhinolaryngol. 1997;254:417–21.
Liu M, Lawson G, Delos M, Jamart J, Chatelain B, Remacle M, Marbaix E. Prognostic value of cell proliferation markers, tumour suppressor proteins and cell adhesion molecules in primary squamous cell carcinoma of the larynx and hypopharynx. Eur Arch Otorhinolaryngol. 2003;260:28–34.
Rittling SR, Chambers AF. Role of osteopontin in tumour progression. Br J Cancer. 2004;90:1877–81.
Zitzmann S, Ehemann V, Schwab M. Arginine-glycine-aspartic acid (RGD)-peptide binds to both tumor and tumor-endothelial cells in vivo. Cancer Res. 2002;62(18):5139–43.
Acknowledgments
I would like to express my gratefulness to my chief adviser, Prof. JG LU, for his patience, encouragement and professional guidance throughout the whole project. Besides, I would like to thank all my friends for their help and advice. Finally, I would like to have sincere thanks to all volunteers who took part in the investigation. Without their participation, this project might not have been made possible and successful.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Y., Li, L., Wang, J.T. et al. Elevated content of osteopontin in plasma and tumor tissues of patients with laryngeal and hypopharyngeal carcinoma associated with metastasis and prognosis. Med Oncol 29, 1429–1434 (2012). https://doi.org/10.1007/s12032-011-0012-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-011-0012-z