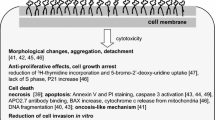
Abstract
Glycosphingolipids are amphipathic molecules composed of hydrophilic oligosaccharide chain and a hydrophobic ceramide part, located primarily in the membrane microdomains of animal cells. Their oligosaccharide chains make them excellent candidates for the cell surface recognition molecules. Natural glycosphingolipid, globotriaosylceramide (Gal α1-4, Gal β1-4, Glc β1-1, ceramide), is also called CD77 and its expression was previously associated with proliferating centroblasts undergoing somatic hypermutation, but it has been demonstrate that globotriaosylceramide is not a reliable marker to discriminate human centroblasts from centrocytes. Globotriaosylceramide constitutes rare P k blood group antigen on erythrocytes, and it is also known as Burkitt’s lymphoma antigen. On endothelial cells, globotriaosylceramide plays as the receptor for bacterial toxins of the Shiga family, also called verotoxins. Precise biological function and significance of globotriaosylceramide expression on endothelial cells remains to be the subject of many studies and it is believed globotriaosylceramide represents an example of a glycolipid antigen able to transduce a signal leading to apoptosis. In past decade, cancer researches put a great afford in determining new therapeutic agents such as bacterial toxins against tumor malignancies. Reports have demonstrated that verotoxin-1 induces apoptosis in solid tumor cell lines expressing globotriaosylceramide such as astrocytoma, renal cell carcinoma, colon cancer and breast cancer due to verotoxin-1 high specificity and apoptosis-inducing properties, and therefore, it is suggested to be an anticancer agent. Verotoxins have been investigated weather they could reduce treatment side-effects and toxicity to normal tissues and become a new oncological tool in cancer labeling.
Similar content being viewed by others
References
Hakomori SI. Cell adhesion/recognition and signal transduction through glycosphingolipid microdomain. Glycoconj J. 2000;17:143–51.
Sonnino S, Prinetti A, Mauri L, Chigorno V, Tettamanti G. Dynamic and structural properties of sphingolipids as driving forces for the formation of membrane domains. Chem Rev. 2006;106:2111–25.
Fantini J, Maresca M, Hammache D, Yahi N, Delézay O. Glycosphingolipid (GSL) microdomains as attachment platforms for host pathogens and their toxins on intestinal epithelial cells: activation of signal transduction pathways and perturbations of intestinal absorption and secretion. Glycoconj J. 2001;17:173–9.
Stults CL, Sweeley CC, Macher BA. Glycosphingolipids: structure, biological source, and properties. Method Enzymol. 1989;179:167–214.
Schnaar RL. Glycosphingolipids in cell surface recognition. Glycobiology. 1991;1:477–85.
Lingwood CA. Role of verotoxin receptors in pathogenesis. Trends Microbiol. 1996;4:147–53.
Konowalchuk J, Dickie N, Stavric S, Speirs JI. Properties of an Escherichia coli cytotoxin. Infect Immun. 1978;20:575–7.
Sandvig K. Shiga toxins. Toxicon. 2001;39:1629–35.
Boyd B, et al. Lipid modulation of glycolipid receptor function. Eur J Biochem. 1994;223:873–8.
Kiarash A, et al. Glycosphingolipid receptor function is modified by fatty acid content. J Biol Chem. 1994;269:11138–46.
Lingwood CA. Aglycone modulation of glycolipid receptor function. Glycoconj J. 1996;13:495–503.
Arab S, Lingwood CA. Intracellular targeting of the endoplasmic reticulum/nuclear envelope by retrograde transport may determine cell hypersensitivity to verotoxin via globotriaosyl ceramide fatty isoform traffic. J Cell Physiol. 1998;177:646–60.
Ling H, et al. Structure of the shiga-like toxin I B-pentamer complexed with an analogue of its receptor Gb3. Biochemistry. 1998;37:1777–88.
Sandvig K, van Deurs B. Entry of ricin and Shiga toxin into cells: molecular mechanisms and medical perspectives. EMBO J. 2000;19:5943–50.
Garred O, van Deurs B, Sandvig K. Furin-induced cleavage and activation of Shiga toxin. J Biol Chem. 1995;270:10817–21.
Endo Y, et al. Site of action of a Vero toxin (VT2) from Escherichia coli O157:H7 and Shiga toxin on eukaryotic ribosomes. Eur J Biochem. 1988;171:45–50.
Mangeney M, et al. Apoptosis induced in Burkitt’s lymphoma cells via Gb3/CD77, a glycolipid antigen. Cancer Res. 1993;53:5314–9.
Fan J, Sammalkorpi M, Haataja M. Formation and regulation of lipid microdomains in cell membranes: theory, modeling, and speculation. FEBS Lett. 2010;584:1678–84.
Gaus K, Chklovskaia E, Fazekas de Groth B, Jessup W, Harder T. Condensation of the plasma membrane at the site of T lymphocyte activation. J Cell Biol. 2005;171:121–31.
Tavano R, et al. CD28 interaction with filamin-A controls lipid raft accumulation at the T-cell immunological synapse. Nat Cell Biol. 2006;8:1270–6.
Chinnapen DJ, Chinnapen H, Saslowsky D, Lencer WI. Rafting with cholera toxin: endocytosis and trafficking from plasma membrane to ER. FEMS Microbiol Lett. 2007;266:129–37.
Kalisiak A, Minniti JG, Oosterwijk E, Old LJ, Scheinberg DA. Neutral glycosphingolipid expression in B-cell neoplasms. Int J Cancer. 1991;49:837–45.
Sandvig K, et al. Pathways followed by ricin and Shiga toxin into cells. Histochem Cell Biol. 2002;117:131–41.
Bonifacino JS, Rojas R. Retrograde transport from endosomes to the trans-Golgi network. Nat Rev Mol Cell Biol. 2006;7:568–79.
Römer W, et al. Shiga toxin induces tubular membrane invaginations for its uptake into cells. Nature. 2007;450:670–5.
Mori T, et al. Globotriaosyl ceramide (CD77/Gb3) in the glycolipidenriched membrane domain participates in B-cell receptor-mediated apoptosis by regulating Lyn kinase activity in human B cells. Exp Hematol. 2000;28:1260–8.
Katagiri YU, et al. Activation of Src family kinase yes induced by Shiga toxin binding to globotriaosyl ceramide (Gb3/CD77) in low density, detergent-insoluble microdomains. J Biol Chem. 1999;274:35278–82.
Falguières T, et al. Targeting of Shiga toxin B-subunit to retrograde transport route in association with detergent resistant membranes. Mol Biol Cell. 2001;12:2453–68.
Kovbasnjuk O, et al. Role of lipid rafts in Shiga toxin 1 interaction with the apical surface of Caco-2 cells. J Cell Sci. 2001;114:4025–31.
Lencer WI, Saslowsky D. Raft trafficking of AB5 subunit bacterial toxins. Biochim Biophys Acta. 2005;1746:314–21.
Tam P, Lingwood C. Membrane-cytosolic translocation of verotoxin A1-subunit in target cells. Microbiology. 2007;153:2700–10.
Römer W, et al. Actin dynamics drive membrane reorganization and scission in clathrin-independent endocytosis. Cell. 2010;140:540–53.
Binnington B, Lingwood D, Nutikka A, Lingwood CA. Effect of globotriaosyl ceramide fatty acid a-hydroxylation on the binding by verotoxin 1 and verotoxin 2. Neurochem Res. 2002;27:807–13.
Newburg D, et al. Susceptibility to hemolytic-uremic syndrome relates to erythrocyte glycosphingolipid patterns. J Infect Dis. 1993;168:476–9.
Lingwood CA. Verotoxin/globotriaosyl ceramide recognition: angiopathy, angiogenesis and antineoplasia. Biosci Rep. 1999;19:345–54.
Kiguchi K, et al. Characteristic expression of globotriaosyl ceramide in human ovarian carcinomaderived cells with anticancer drug resistance. Cancer Sci. 2006;97:1321–6.
Sandvig K, et al. Retrograde transport from the golgi complex to the ER of both Shiga toxin and the nontoxic Shiga B-fragment is regulated by butyric acid and cAMP. J Cell Biol. 1994;126:53–64.
Tam P, et al. Differential intracellular trafficking and binding of verotoxin 1 and verotoxin 2 to globotriaosylceramide-containing lipid assemblies. J Cell Physiol. 2008;216:750–63.
Burger K, Gimpl G, Fahrenholz F. Regulation of receptor function by cholesterol. Cell Mol Life Sci. 2000;57:1577–92.
Chark D, Nutikka A, Trusevych N, Kuzmina J, Lingwood C. Differential carbohydrate epitope recognition of globotriaosyl ceramide by verotoxins and a monoclonal antibody. Eur J Bioch. 2004;271:405–17.
Smith DC, et al. The association of Shiga-like toxin with detergent-resistant membranes is modulated by glucosylceramide and is an essential requirement in the endoplasmic reticulum for a cytotoxic effect. Mol Biol Cell. 2006;17:1375–87.
Hoey DEE, Currie C, Lingwood CA, Gally DL, Smith DGE. Binding of verotoxin 1 to primary intestinal epithelial cells expressing Gb3 results in trafficking of toxin to lysosomal compartments. Cell Microbiol. 2003;5:85–97.
Khine AA, Firtel M, Lingwood CA. CD77-dependent retrograde transport of CD19 to the nuclear membrane: functional relationship between CD77 and CD19 during germinal center B-cell apoptosis. J Cell Physiol. 1998;176:281–92.
Maloney MD, Lingwood CA. CD19 Has a potential CD77 (Globotriaosyl ceramide)-binding site with sequence similarity to verotoxin B-subunits: implications of molecular mimicry for B cell adhesion and enterohemorrhagic Escherichia coli pathogenesis. J Exp Med. 1994;180:191–201.
Obrig TG, et al. Direct cytotoxic action of Shiga toxin on human vascular endothelial cells. Infect Immun. 1988;56:2373–8.
Ohmi K, Kiyokawa N, Takeda T, Fujimoto J. Human microvascular endothelial cells are strongly sensitive to Shiga toxin. Biochem Biophys Res Commun. 1998;251:137–41.
Holgersson J, Jovall PA, Breimer ME. Glycosphingolipids of human large intestine: detailed structural characterization with special reference to blood group compounds and bacterial receptor structures. J Biochem. 1991;110:120–31.
Schuller S, Frankel G, Phillips AD. Interaction of Shiga toxin from Escherichia coli with human intestinal epithelial cell lines and explants: Stx2 induces epithelial damage in organ culture. Cell Microbiol. 2004;6:289–301.
Ramegowda B, Samuel JE, Tesh VL. Interaction of Shiga toxins with human brain microvascular endothelial cells: cytokines as sensitizing agents. J Infect Dis. 1999;180:1205–13.
Eisenhauer PE, et al. Tumor necrosis factor alpha increases human cerebral endothelial cell Gb3 and sensitivity to Shiga toxin. Infect Immun. 2001;69:1889–94.
Stricklett PK, Hughes AK, Ergonul Z, Kohan DE. Molecular basis for up-regulation by inflammatory cytokines of Shiga toxin 1 cytotoxicity and globotriaosylceramide expression. J Infect Dis. 2002;186:976–82.
Ergonul Z, Hughes AK, Kohan DE. Induction of apoptosis of human brain microvascular endothelial cells by Shiga toxin. J Infect Dis. 2003;187:154–8.
Uchida H, et al. Shiga toxins induce apoptosis in pulmonary epithelium–derived cells. J Infect Dis. 1999;180:1902–11.
Takahashi K, Funata N, Ikuta F, Sato S. Neuronal apoptosis and inflammatory responses in the central nervous system of a rabbit treated with Shiga toxin-2. J Neuroinflamm. 2008;5:11–23.
De Rosa MF, et al. Inhibition of multidrug resistance by adamantylgb3, a globotriasylceramide analog. J Biol Chem. 2008;283:4501–11.
Bielaszewska M, Karch H. Consequences of enterohaemorrhagic Escherichia coli infection for the vascular endothelium. Thromb Haemost. 2005;94:312–8.
Nakao H, Takeda T. Escherichia coli Shiga toxin. J Nat Toxins. 2000;9:299–313.
Karch H. The role of virulence factors in enterohemorrhagic Escherichia coli (EHEC)-associated hemolytic: uremic syndrome. Semin Thromb Hemost. 2001;27:207–13.
Forsyth KD, et al. Neutrophil-mediated endothelial injury in haemolytic uraemic syndrome. Lancet. 1989;2:411–4.
Morigi M, et al. Verotoxin-1-induced up-regulation of adhesive molecules renders microvascular endothelial cells thrombogenic at high shear stress. Blood. 2001;98:1828–35.
Ching JC, Jones NL, Ceponis PJ, Karmali MA, Sherman PM. Escherichia coli shiga-like toxins induce apoptosis and cleavage of poly (ADP-ribose) polymerase via in vitro activation of caspases. Infect Immun. 2002;70:4669–77.
Kiyokawa N, et al. Activation of the caspase cascade during Stx1-induced apoptosis in Burkitt’s lymphoma cells. J Cell Biochem. 2001;81:128–42.
Tétaud C, et al. Two distinct Gb3/CD77 signaling pathways leading to apoptosis are triggered by anti-Gb3/CD77 mAb and verotoxin-1. J Biol Chem. 2003;278:45200–8.
Garibal J, Hollville É, Renouf B, Tétaud C, Wiels J. Caspase-8-mediated cleavage of Bid and protein phosphatase 2A-mediated activation of Bax is necessary for Verotoxin-1-induced apoptosis in Burkitt’s lymphoma cells. Cell Signal. 2001;22:467–75.
Lee SY, Cherla RP, Caliskan I, Tesh VL. Shiga toxin 1 induces apoptosis in the human myelogenous leukemia cell line THP-1 by a caspase-8-dependent, tumor necrosis factor-independent mechanism. Infect Immun. 2008;73:5115–26.
Erwert RD, et al. Shiga toxin induces decreased expression of the anti-apoptotic protein Mcl-1 concomitant with the onset of endothelial apoptosis. Microb Pathog. 2003;35:87–93.
Fujii J, et al. Rapid apoptosis induced by Shiga toxin in HeLa cells. Infect Immun. 2003;71:2724–35.
Gariepy J. The use of Shiga-like toxin 1 in cancer therapy. Crit Rev Oncol Hematol. 2001;39:99–106.
LaCasse EC, et al. Shiga-like toxin-1 receptor on human breast cancer, lymphoma, myeloma and absence from CD34(+) hematopoietic stem cells: implications for ex vivo tumor purging and autologous stem cell transplantation. Blood. 1999;94:2901–10.
Salhia B, Rutka JT, Lingwood C, Nutikka A, Van Furth WR. The treatment of malignant meningioma with verotoxin. Neoplasia. 2002;4:304–11.
Murray LJ, Habeshaw JA, Wiels J, Greaves MF. Expression of Burkitt lymphoma-associated antigen (defined by the monoclonal antibody 38.13) on both normal and malignant germinal-centre B cells. Int J Cancer. 1985;36:561–5.
Ohyama C, et al. Changes in glycolipid expression in human testicular tumor. Int J Cancer. 1990;45:1040–4.
Johansson D, et al. Verotoxin-1 induction of apoptosis in Gb3- expressing human glioma cell lines. Cancer Biol Ther. 2006;5:1211–7.
Arab S, et al. Expression of the verotoxin receptor glycolipid, globotriaosylceramide, in ovarian hyperplasias. Oncol Res. 1997;9:553–63.
Fredman P, Hedberg K, Brezicka T. Gangliosides as therapeutic targets for cancer. BioDrugs. 2003;17:155–67.
Kovbasnjuk O, et al. The glycosphingolipid globotriaosylceramide in the metastatic transformation of colon cancer. Proc Natl Acad Sci USA. 2005;102:19087–92.
Johannes L, Decaudin D. Protein toxins: intracellular trafficking for targeted therapy. Gene Ther. 2005;12:1360–8.
Janssen KP, et al. In vivo tumor targeting using a novel intestinal pathogen-based delivery approach. Cancer Res. 2006;66:7230–6.
Ludwig K, et al. Antibody response to Shiga toxins Stx2 and Stx1 in children with enteropathic hemolytic-uremic syndrome. J Clin Microbiol. 2001;39:2272–9.
Levine MM, et al. Antibodies to shiga holotoxin and to two synthetic peptides of the B subunit in sera of patients with Shigella dysenteriae 1 dysentery. J Clin Microbiol. 1992;30:1636–41.
O’Brien AD, et al. Shiga toxin: biochemistry, genetics, mode of action, and role in pathogenesis. Curr Top Microbiol Immunol. 1992;180:65–94.
Vingert B, et al. The Shiga toxin B-subunit targets antigen in vivo to dendritic cells and elicits anti-tumor immunity. Eur J Immunol. 2006;36:1124–35.
Haicheur N, et al. Vt B-subunit of Shiga toxin fused to a tumor antigen elicits CTL and targets dendritic cells to allow MHC class I restricted presentation of peptides derived from exogenous antigens. J Immunol. 2000;165:3301–8.
Wang H, Griffiths MN, Burton DR, Ghazal P. Rapid antibody responses by low-dose, single-step, dendritic cell-targeted immunization. Proc Natl Acad Sci USA. 2000;97:847–85.
Haicheur N, et al. The B subunit of Shiga toxin coupled to fullsize antigenic protein elicits humoral and cell-mediated immune responses associated with a Th1-dominant polarization. Int Immunol. 2003;15:1–11.
Falguières T. Human colorectal tumors and metastases express Gb3 and can be targeted by an intestinal pathogen-based delivery tool. Mol Cancer Ther. 2008;7:2498–508.
Viel T, et al. In vivo tumor targeting by the B-subunit of shiga toxin. Mol Imaging. 2008;7:239–47.
Rutjes NW, Binnington BA, Smith CR, Maloney MD, Lingwood CA. Differential tissue targeting and pathogenesis of verotoxins 1 and 2 in the mouse animal model. Kidney Int. 2002;62:832–45.
Imberty A, Wimmerová M, Mitchell EP, Gilboa-Garber N. Structures of the lectins from Pseudomonas aeruginosa: insight into the molecular basis for host glycan recognition. Microbes Infect. 2004;6:221–8.
Blanchard B, et al. Structural basis of the preferential binding for globo-series glycosphingolipids displayed by Pseudomonas aeruginosa lectin I. Mol Biol. 2008;383:837–53.
Distler U, et al. Shiga toxin receptor Gb3Cer/CD77: tumor-association and promising therapeutic target in pancreas and colon cancer. PLoS ONE. 2009;4(8):e6813.
Schuller S, Heuschkel R, Torrente F, Kaper JB, Phillips AD. Shiga toxin binding in normal and inflamed human intestinal mucosa. Microbes Infect. 2007;9:35–9.
Ishitoya S, et al. Verotoxin induces rapid elimination of human renal tumor xenografts in SCID mice. J Urol. 2004;171:1309–13.
Arab S, Rutka J, Lingwood C. Verotoxin induces apoptosis and the complete, rapid, long-term elimination of human astrocytoma xenografts in nude mice. Oncol Res. 1999;11:33–9.
Heath-Engel HM, Lingwood CA. Verotoxin sensitivity of ECV304 cells in vitro and in vivo in a xenograft tumour model: VT1 as a tumour neovascular marker. Angiogenesis. 2003;6:129–41.
Johansson D, et al. Expression of verotoxin-1 receptor Gb3 in breast cancer tissue and verotoxin-1 signal transduction to apoptosis. BMC Cancer. 2009;9:67–76.
Bornkamm GW, Polack A, Eick D, Berger R, Lenoir GM. Chromosome translocations and Epstein-Barr virus in Burkitt’s lymphoma. Onkologie. 1987;10:196–204.
Jaffe ES, et al. Burkitt’s lymphoma: a single disease with multiple variants. The World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. Blood. 1999;3:1124.
Gordon J, et al. CD40 ligand, Bcl-2, and Bcl-xL spare group I Burkitt lymphoma cells from CD77-directed killing via Verotoxin-1B chain but fail to protect against the holotoxin. Cell Death Differ. 2000;7:785–94.
Cohen A, Hannigan GE, Williams BRG, Lingwood CA. Roles of globotriosyl- and galabiosylceramide in verotoxin binding and high affinity interferon receptor. J Biol Chem. 1987;262:17088–91.
Wiels J, Fellous M, Tursz T. Monoclonal antibody against a Burkitt lymphoma associated antigen. Proc Natl Acad Sci USA. 1981;78:6485–9.
Sakthivel R, Christensson B, Ehlin-Henriksson B, Klein G. Immunophenotypic characterization of follicle-center-cell-derived non-Hodgkin’s lymphomas. Int J Cancer. 1989;43:624–30.
Taga S, et al. Intracellular signalling events in CD77-mediated apoptosis of Burkitt’s lymphoma cells. Blood. 1997;90:2757–67.
Grafton G, Goodall M, Gregory CD, Gordon J. Mechanisms of antigen receptor-dependent apoptosis in human B lymphoma cell lines probed with a panel of 27 monoclonal antibodies. Cell Immunol. 1997;182:45–56.
Michael JM, Lavin MF, Watters DJ. Resistance to radiation-induced apoptosis in Burkitt’s lymphoma cells is associated with defective ceramide signaling. Cancer Res. 1997;57:3600–5.
Furukawa K, et al. Expression of the Gb3/CD77 synthase gene in megakaryoblastic leukemia cells. J Biol Chem. 2002;277:11247–54.
Zhang X-g, et al. Reproducible obtaining of human myeloma cell lines as a model for tumor stem cell study in human multiple myeloma. Blood. 1994;83:3654–63.
Szczepek AJ, et al. A high frequency of circulating B cells share clonotypic IgH VDJ rearrangements with autologous bone marrow plasma cells in multiple myeloma, as measured by single cell and in situ RT-PCR. Blood. 1998;92:2844–55.
Bechtel D, Kurth J, Unkel C, Ku¨ppers R. Transformation of BCR-deficient germinal-center B cells by EBV supports a major role of the virus in the pathogenesis of Hodgkin and posttransplantation lymphomas. Blood. 2005;106:4345–50.
Arbus GS, et al. Verotoxin targets lymphoma infiltrates of patients with post-transplant lymphoproliferative disease. Leukemia Res. 2000;24:857–64.
Craxton A, Chuang PI, Shu G, Harlan JM, Clark EA. The CD40-inducible Bcl-2 family member A1 protects B Cells from antigen receptor-mediated apoptosis. Cell Immunol. 2000;200:56–62.
Li T, Ramirez K, Palacios R. Distinct patterns of Fas cell surface expression during development of T- or B-lymphocyte lineages in normal, scid, and mutant mice lacking or overexpressing p53, bcl-2, or rag-2 genes. Cell Growth Differ. 1996;7:107–14.
Ghia P, et al. Unbalanced expression of Bcl-2 family proteins in follicular lymphoma: contribution of CD40 signaling in promoting survival. Blood. 1998;91:224–51.
Holder MJ, et al. Suppression of apoptosis in normal and neoplastic human B lymphocytes by CD40 ligand is independent of Bcl-2 induction. Eur J Immunol. 1993;23:2368–71.
Traulle C, Coiffier B. Evolving role of rituximab in the treatment of patients with non-Hodgkin’s lymphoma. Future Oncol. 2005;1:297–306.
Béniguel L, et al. identification of germinal center B cells in blood from HIV-infected drug-naive individuals in central Africa. Clin Dev Immunol. 2004;11:23–7.
Ramkumar S, Sakac D, Binnington B, Branch DR, Lingwood CA. Induction of HIV-1 resistance: cell susceptibility to infection is an inverse function of globotriaosyl ceramide levels. Glycobiology. 2009;19:76–82.
Acknowledgment
This work resulted from scientific project “Pathobiochemistry of glycosphingolipid antigens” carried out by support of Ministry of Science, Education and Sports, Republic of Croatia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Đevenica, D., Čikeš Čulić, V., Vuica, A. et al. Biochemical, pathological and oncological relevance of Gb3Cer receptor. Med Oncol 28 (Suppl 1), 675–684 (2011). https://doi.org/10.1007/s12032-010-9732-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-010-9732-8