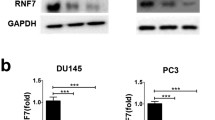
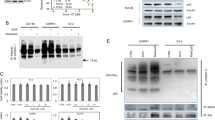
Abstract
p53 is the most frequently mutated tumor suppressor gene in human cancer. Recent studies have indicated that p53 mutants not only lose tumor suppression activity but also gain novel oncogenic functions that contribute to tumor malignancy. In this study, we explored mutant p53 as a target for novel anti-cancer treatment in prostate cancer. Using the DU145 human androgen-independent prostate cancer cell line, we show that silencing of mutant p53 gene by RNA interference led to significant inhibition of cell viability and growth, which was associated with cell cycle arrest at G1 and G2/M phase, and ultimately induced massive apoptosis. Mechanistically, p53-siRNA inhibited phosphatidylinositol 3′-kinase/Akt signaling pathway, which might be responsible for the reduced proliferation and apoptosis induction. These findings suggest that RNA interference targeting mutant p53 may serve as a novel therapeutic strategy for the treatment of androgen-independent prostate cancer.





Similar content being viewed by others
References
Jemal A, Siegel R, Ward E, Hao Y, Xu J, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49.
Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–31.
Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53.
Milner J, Medcalf EA, Cook AC. Tumor suppressor p53: analysis of wild-type and mutant p53 complexes. Mol Cell Biol. 1991;11:12–9.
Milner J, Medcalf EA. Cotranslation of activated mutant p53 with wild type drives the wild-type p53 protein into the mutant conformation. Cell. 1991;65:765–74.
Aas T, Borresen AL, Geisler S, Smith-Sorensen B, Johnsen H, et al. Specific P53 mutations are associated with de novo resistance to doxorubicin in breast cancer patients. Nat Med. 1996;2:811–4.
Blandino G, Levine AJ, Oren M. Mutant p53 gain of function: differential effects of different p53 mutants on resistance of cultured cells to chemotherapy. Oncog. 1999;18:477–85.
Navone NM, Troncoso P, Pisters LL, Goodrow TL, Palmer JL, et al. p53 protein accumulation and gene mutation in the progression of human prostate carcinoma. J Natl Cancer Inst. 1993;85:1657–69.
Perryman LA, Blair JM, Kingsley EA, Szymanska B, Ow KT, et al. Over-expression of p53 mutants in LNCaP cells alters tumor growth and angiogenesis in vivo. Biochem Biophys Res Commun. 2006;345:1207–14.
Che M, DeSilvio M, Pollack A, Grignon DJ, Venkatesan VM, et al. Prognostic value of abnormal p53 expression in locally advanced prostate cancer treated with androgen deprivation and radiotherapy: a study based on RTOG 9202. Int J Radiat Oncol Biol Phys. 2007;69:1117–23.
Carroll AG, Voeller HJ, Sugars L, Gelmann EP. p53 oncogene mutations in three human prostate cancer cell lines. Prostate. 1993;23:123–34.
Xie L, Zheng X, Qin J, Chen Z, Jin Y, et al. Role of PI3-kinase/Akt signalling pathway in renal function and cell proliferation after renal ischaemia/reperfusion injury in mice. Nephrology (Carlton). 2006;11:207–12.
Ma LL, Sun WJ, Wang Z, Zh GY, Li P, et al. Effects of silencing of mutant p53 gene in human lung adenocarcinoma cell line Anip973. J Exp Clin Cancer Res. 2006;25:585–92.
Ivana Scovassi A, Diederich M. Modulation of poly(ADP-ribosylation) in apoptotic cells. Biochem Pharmacol. 2004;68:1041–7.
Sarker D, Reid AH, Yap TA, de Bono JS. Targeting the PI3 K/AKT pathway for the treatment of prostate cancer. Clin Cancer Res. 2009;15:4799–805.
Slater EP, Stubig T, Lau QC, Achenbach TV, Rapp UR, et al. C-Raf controlled pathways in the protection of tumor cells from apoptosis. Int J Cancer. 2003;104:425–32.
Fridman JS, Lowe SW. Control of apoptosis by p53. Oncogene. 2003;22:9030–40.
Li R, Sutphin PD, Schwartz D, Matas D, Almog N, et al. Mutant p53 protein expression interferes with p53-independent apoptotic pathways. Oncogene. 1998;16:3269–77.
Bergamaschi D, Gasco M, Hiller L, Sullivan A, Syed N, et al. p53 polymorphism influences response in cancer chemotherapy via modulation of p73-dependent apoptosis. Cancer Cell. 2003;3:387–402.
Wolf D, Harris N, Rotter V. Reconstitution of p53 expression in a nonproducer Ab-MuLV-transformed cell line by transfection of a functional p53 gene. Cell. 1984;38:119–26.
Ghosh PM, Malik S, Bedolla R, Kreisberg JI. Akt in prostate cancer: possible role in androgen-independence. Curr Drug Metab. 2003;4:487–96.
Bookstein R, MacGrogan D, Hilsenbeck SG, Sharkey F, Allred DC. p53 is mutated in a subset of advanced-stage prostate cancers. Cancer Res. 1993;53:3369–73.
Brosh R, Rotter V. When mutants gain new powers: news from the mutant p53 field. Nat Rev Cancer. 2009;9:701–13.
Dong P, Xu Z, Jia N, Li D, Feng Y. Elevated expression of p53 gain-of-function mutation R175H in endometrial cancer cells can increase the invasive phenotypes by activation of the EGFR/PI3 K/AKT pathway. Mol Cancer. 2009;8:103.
Gesbert F, Sellers WR, Signoretti S, Loda M, Griffin JD. BCR/ABL regulates expression of the cyclin-dependent kinase inhibitor p27Kip1 through the phosphatidylinositol 3-Kinase/AKT pathway. J Biol Chem. 2000;275:39223–30.
Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–511.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, H., Mao, Q., Lin, Y. et al. RNA interference targeting mutant p53 inhibits growth and induces apoptosis in DU145 human prostate cancer cells. Med Oncol 28 (Suppl 1), 381–387 (2011). https://doi.org/10.1007/s12032-010-9679-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-010-9679-9