Abstract
The aim of this study was to evaluate the frequency (as qualitative analysis) and level (as quantitative analysis) of promoter hypermethylation of four genes, P16, TSHR, RASSF1A and RARβ2, and to assess their diagnostic or prognostic values in papillary thyroid tumors. Fifty formalin-fixed paraffin-embedded (FFPE) samples consisting of 25 malignant tumors and 25 non-malignant thyroid tumors were analyzed using COBRA method. Promoter hypermethylation of P16, TESH, RASSF1A and RARB2 genes was noted not only in 10, 7, 19 and 13 cases of malignant tumors, but also it was detected in 7, 11, 23 and 8 cases of benign tumors, respectively, limiting its diagnostic usefulness. The quantitative hypermethylation level was significantly higher in malignant tumors compared to benign tumors for P16 (P < 0.004), TSHR (P < 0.006) and RASSF1A (P < 0.001), but the methylation level of RARβ2 (P < 0.31) showed considerable overlap between the two groups. The mean levels of hypermethylation of P16, TSHR and RASSF1A genes were significantly higher in malignant papillary thyroid tumors compared to benign tumors and by choosing the appropriate cutoff for each gene, we could distinguish 9, 9 and 8 PTCs from 25 cases by P16, RASSF1A and TSHR methylation analysis, respectively. According to our results, these three genes, in combination, may be useful as molecular markers. The findings of present study imply that the P16, TSHR and RASSF1A gene promoter hypermethylation may play important roles in the pathogenesis of PTC and can be a potential biomarker for selecting patients with PTC.


Similar content being viewed by others
References
Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993;328(8):553–9.
Belfiore A, La Rosa GL. Fine-needle aspiration biopsy of the thyroid. Endocrinol Metab Clin North Am. 2001;30(2):361–400.
McCaffrey TV. Evaluation of the thyroid nodule. Cancer Control. 2000;7(3):223–8.
Mohammadi-asl J, Larijani B, Khorgami Z, Tavangar SM, Haghpanah V, Mehdipour P. Prevalence of BRAFV600E mutation in iranian patients with papillary thyroid carcinoma: a single-center study. J Appl Sci. 2009;9(19):3593–7.
Smith JA, Fan CY, Zou C, Bodenner D, Kokoska MS. Methylation status of genes in papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg. 2007;133(10):1006–11.
Fryknas M, Wickenberg-Bolin U, Goransson H, Gustafsson MG, Foukakis T, Lee JJ, et al. Molecular markers for discrimination of benign and malignant follicular thyroid tumors. Tumor Biol. 2006;27(4):211–20.
Haghpanah V, Shooshtarizadeh P, Heshmat R, Larijani B, Tavangar SM. Immunohistochemical analysis of survivin expression in thyroid follicular adenoma and carcinoma. Appl Immunohistochem Mol Morphol. 2006;14(4):422–5.
Herman JG. Epigenetic changes in cancer and preneoplasia. Cold Spring Harb Symp Quant Biol. 2005;70:329–33.
Robertson KD, Jones PA. Tissue-specific alternative splicing in the human INK4a/ARF cell cycle regulatory locus. Oncogene. 1999;18(26):3810–20.
Lam AK, Lo CY, Leung P, Lang BH, Chan WF, Luk JM. Clinicopathological roles of alterations of tumor suppressor gene p16 in papillary thyroid carcinoma. Ann Surg Oncol. 2007;14(5):1772–9.
Boltze C, Zack S, Quednow C, Bettge S, Roessner A, Schneider-Stock R. Hypermethylation of the CDKN2/p16INK4A promotor in thyroid carcinogenesis. Pathol Res Pract. 2003;199(6):399–404.
Hoque MO, Rosenbaum E, Westra WH, Xing M, Ladenson P, Zeiger MA, et al. Quantitative assessment of promoter methylation profiles in thyroid neoplasms. J Clin Endocrinol Metab. 2005;90(7):4011–8.
Schagdarsurengin U, Gimm O, Dralle H, Hoang-Vu C, Dammann R. CpG island methylation of tumor-related promoters occurs preferentially in undifferentiated carcinoma. Thyroid. 2006;16(7):633–42.
Kopp P. The TSH receptor and its role in thyroid disease. Cell Mol Life Sci. 2001;58(9):1301–22.
Xing M, Usadel H, Cohen Y, Tokumaru Y, Guo Z, Westra WB, et al. Methylation of the thyroid-stimulating hormone receptor gene in epithelial thyroid tumors: a marker of malignancy and a cause of gene silencing. Cancer Res. 2003;63(9):2316–21.
Shivakumar L, Minna J, Sakamaki T, Pestell R, White MA. The RASSF1A tumor suppressor blocks cell cycle progression and inhibits cyclin D1 accumulation. Mol Cell Biol. 2002;22(12):4309–18.
Tommasi S, Dammann R, Zhang Z, Wang Y, Liu L, Tsark WM, et al. Tumor susceptibility of Rassf1a knockout mice. Cancer Res. 2005;65(1):92–8.
Schagdarsurengin U, Gimm O, Hoang-Vu C, Dralle H, Pfeifer GP, Dammann R. Frequent epigenetic silencing of the CpG island promoter of RASSF1A in thyroid carcinoma. Cancer Res. 2002;62(13):3698–701.
Nakamura N, Carney JA, Jin L, Kajita S, Pallares J, Zhang H, et al. RASSF1A and NORE1A methylation and BRAFV600E mutations in thyroid tumors. Lab Invest. 2005;85(9):1065–75.
Soprano DR, Qin P, Soprano KJ. Retinoic acid receptors and cancers. Annu Rev Nutr. 2004;24:201–21.
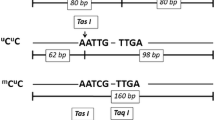
Xiong Z, Laird PW. COBRA: a sensitive and quantitative DNA methylation assay. Nucleic Acids Res. 1997;25(12):2532–4.
Konishi N, Nakamura M, Kishi M, Nishimine M, Ishida E, Shimada K. Heterogeneous methylation and deletion patterns of the INK4a/ARF locus within prostate carcinomas. Am J Pathol. 2002;160(4):1207–14.
Hu S, Ewertz M, Tufano RP, Brait M, Carvalho AL, Liu D, et al. Detection of serum deoxyribonucleic acid methylation markers: a novel diagnostic tool for thyroid cancer. J Clin Endocrinol Metab. 2006;91(1):98–104.
Ishida E, Nakamura M, Shimada K, Higuchi T, Takatsu K, Yane K, et al. DNA hypermethylation status of multiple genes in papillary thyroid carcinomas. Pathobiology. 2007;74(6):344–52.
Acknowledgments
Authors would like to thank School of Medicine, Tehran University of Medical Sciences for the financial supports.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohammadi-asl, J., Larijani, B., Khorgami, Z. et al. Qualitative and quantitative promoter hypermethylation patterns of the P16, TSHR, RASSF1A and RARβ2 genes in papillary thyroid carcinoma. Med Oncol 28, 1123–1128 (2011). https://doi.org/10.1007/s12032-010-9587-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-010-9587-z