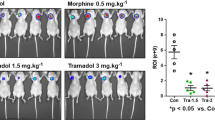
Abstract
Breast cancer is the leading cause of death among women, and morphine is used to relieve the pain of patients with cancer. The data on the effects of morphine on tumour growth and angiogenesis are contradictory. We determined in mouse breast cancer model whether analgesic doses of morphine would affect tumour angiogenesis, and then the correlation between microvessel density (MVD), Doppler sonography (DS) and 99mTc-Tetrofosmin (TF) uptake. Ehrlich ascites tumour cell xenografts, Pgp-negative tumour were divided into two groups: (a) Morphine sulphate [0.714 mg/kg/day (equivalent to 50 mg per day for a 70 kg human)], (b) no-morphine. For the determination of angiogenesis in mice tumour tissue, TF scintigraphy, microvessel density and DS were done. MVD was significantly different between groups (49.4 ± 1.8 vs. 41.8 ± 1.9, morphine and no-morphine groups, respectively, P < 0.001). A strong correlation was found between late uptakes of mass at scintigraphy and degree of angiogenesis in histopathologic examination (r = 0.52, P < 0.01). There was statistically significant inverse correlation between degree of angiogenesis in histopathologic examination and washout ratio of TF (r = 0.40, P < 0.05). The higher values for angiogenesis are related to higher TF reuptake. There was no statistically significant correlation between DS and TF. A strong correlation was found between MVD and grade of DS (r = 0.51, P < 0.01). Our preclinical mice study indicates that morphine at clinically relevant doses stimulates angiogenesis, and angiogenesis triggered of morphine is demonstrated with MVD and DS, but not TF. However, uptake and washout of TF are compared with immunohistochemically assessed morphine-stimulated angiogenesis in tumour tissue.






Similar content being viewed by others
References
Higley B, Smith FW, Smith T, Gemmell HG, Das Gupta P, et al. Technetium-99 m-1, 2-bis[bis(2-ethoxyethyl) phosphino]ethane: human biodistribution, dosimetry and safety of a new myocardial perfusion imaging agent. J Nucl Med. 1993;34:30–8.
Rambaldi PF, Mansi L, Procaccini E, Di Gregorio F, Del Vecchio E. Breast cancer detection with Tc-99 m tetrofosmin. Clin Nucl Med. 1995;20:703–5.
Klain M, Maurea S, Lastoria S, Cuocolo A, Colao A, et al. Technetium-99 m-tetrofosmin imaging of differentiated mixed thyroid cancer. J Nucl Med. 1995;36:2248–51.
Basoglu T, Sahin M, Coskun C, Koparan A, Bernay I, et al. Technetium-99 m-tetrofosmin uptake in malignant lung tumours. Eur J Nucl Med. 1995;22:687–9.
Rodrigues M, Chehne F, Kalinowska W, Berghammer P, Zielinski C, et al. Uptake of 99 mTc-MIBI and 99 mTc-tetrofosmin into malignant versus nonmalignant breast cell lines. J Nucl Med. 2000;41:1495–9.
Liu TJ TJ, Shiau YC, Tsai SC, Wang JJ, Ho ST, et al. Predicting multidrug resistance-related protein and P-glycoprotein expression with technetium-99 m tetrofosmin mammoscintigraphy. Breast. 2003;12:58–62.
Fuster D, Viñolas N, Mallafré C, Pavia J, Martín F, et al. Tetrofosmin as predictors of tumour response. Q J Nucl Med. 2003;47:58–62.
Yoon JH, Bom HS, Song HC, Lee JH, Jaegal YJ. Double-phase Tc-99m sestamibi scintimammography to assess angiogenesis and P-glycoprotein expression in patients with untreated breast cancer. Clin Nucl Med. 1999;24:314–8.
Lickiss JN. Approaching cancer pain relief. Eur J Pain. 2001;5(Suppl A):5–14.
Sueoka N, Sueoka E, Okabe S, Fujiki H. Anti-cancer effects of morphine through inhibition of tumour necrosis factor-alpha release and mRNA expression. Carcinogenesis. 1996;17:2337–41.
Maneckjee R, Minna JD. Opioids induce while nicotine suppresses apoptosis in human lung cancer cells. Cell Growth Differ. 1994;5:1033–40.
Maneckjee R, Minna JD. Opioid and nicotine receptors affect growth regulation of human lung cancer cell lines. Proc Natl Acad Sci USA. 1990;87:3294–8.
Pasi A, Qu BX, Steiner R, Senn HJ, Bär W, et al. Angiogenesis: modulation with opioids. Gen Pharmacol. 1991;22:1077–9.
Hatzoglou A, Ouafik L, Bakogeorgou E, Thermos K, Castanas E. Morphine cross-reacts with somatostatin receptor SSTR2 in the T47D human breast cancer cell line and decreases cell growth. Cancer Res. 1995;55:5632–6.
Hatzoglou A, Bakogeorgou E, Castanas E. The antiproliferative effect of opioid receptor agonists on the T47D human breast cancer cell line, is partially mediated through opioid receptors. Eur J Pharmacol. 1996;296:199–207.
Maneckjee R, Biswas R, Vonderhaar BK. Binding of opioids to human MCF-7 breast cancer cells and their effects on growth. Cancer Res. 1990;50:2234–8.
Kampa M, Bakogeorgou E, Hatzoglou A, Damianaki A, Martin PM, et al. Opioid alkaloids and casomorphin peptides decrease the proliferation of prostatic cancer cell lines (LNCaP, PC3 and DU145) through a partial interaction with opioid receptors. Eur J Pharmacol. 1997;335:255–65.
Tegeder I, Grösch S, Schmidtko A, Häussler A, Schmidt H, et al. G protein-independent G1 cell cycle block and apoptosis with morphine in adenocarcinoma cells: involvement of p53 phosphorylation. Cancer Res. 2003;63:1846–52.
Yeager MP, Colacchio TA. Effect of morphine on growth of metastatic colon cancer in vivo. Arch Surg. 1991;126:454–6.
Sasamura T, Nakamura S, Iida Y, Fujii H, Murata J, et al. Morphine analgesia suppresses tumor growth and metastasis in a mouse model of cancer pain produced by orthotopic tumor inoculation. Eur J Pharmacol. 2002;441:185–91.
Harimaya Y, Koizumi K, Andoh T, Nojima H, Kuraishi Y, et al. Potential ability of morphine to inhibit the adhesion, invasion and metastasis of metastatic colon 26–L5 carcinoma cells. Cancer Lett. 2002;187:121–7.
Simon RH, Arbo TE. Morphine increases metastatic tumor growth. Brain Res Bull. 1986;16:363–7.
Lewis JW, Shavit Y, Terman GW, Nelson LR, Gale RP, et al. Apparent involvement of opioid peptides in stress-induced enhancement of tumor growth. Peptides. 1983;4:635–8.
Sergeeva MG, Grishina ZV, Varfolomeyev SD. Morphine effect on proliferation of normal and tumor cells of immune origin. Immunol Lett. 1993;36:215–8.
Gupta K, Kshirsagar S, Chang L, Schwartz R, Law PY, et al. Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res. 2002;62:4491–8.
Farooqui M, Li Y, Rogers T, Poonawala T, Griffin RJ, et al. COX-2 inhibitor celecoxib prevents chronic morphine-induced promotion of angiogenesis, tumour growth, metastasis and mortality, without compromising analgesia. Br J Cancer. 2007;97:1523–31.
Zhu X, Wu H, Xia J, Zhao M, Xianyu Z. The relationship between (99m)Tc-MIBI uptakes and tumor cell death/proliferation state under irradiation. Cancer Lett. 2002;182:217–22.
Ustun F, Durmus-Altun G, Cukur Z, Altaner S, Berkarda S. Glucose-induced alteration of accumulation of organotechnetium complexes accumulation in Pgp-negative tumor-bearing mice. Cancer Biother Radiopharm. 2009;24:333–8.
Benerjee SK, Zoubime MN, Mullick M, Weston AP, Cherian R, et al. Tumor angiogenesis in chronic pancreatitis and pancreatic adenocarcinoma: impact of K-ras mutations. Pankreas. 2000;20:248–55.
Singleton PA, Lingen MW, Fekete MJ, Garcia JG, Moss J. Methylnaltrexone inhibits opiate and VEGF-induced angiogenesis: role of receptor transactivation. Microvasc Res. 2006;72:3–11.
Arerangaiah R, Chalasani N, Udager AM, Weber ML, Manivel JC, et al. Opioids induce renal abnormalities in tumor-bearing mice. Nephron Exp Nephrol. 2007;105:e80–9.
Freier DO, Fuchs BA. A mechanism of action for morphine-induced immunosuppression: corticosterone mediates morphine-induced suppression of natural killer cell activity. J Pharmacol Exp Ther. 1994;270:1127–33.
Glasel JA. The effects of morphine on cell proliferation. Prog Drug Res. 2000;55:33–80.
Peterson PK, Molitor TW, Chao CC. Mechanisms of morphine-induced immunomodulation. Biochem Pharmacol. 1993;46:343–8.
Ishikawa M, Tanno K, Kamo A, Takayanagi Y, Sasaki K. Enhancement of tumor growth by morphine and its possible mechanism in mice. Biol Pharm Bull. 1993;16:762–6.
Carr DJ, Serou M. Exogenous and endogenous opioids as biological response modifiers. Immunopharmacology. 1995;31:59–71.
Fecho K, Maslonek KA, Dykstra LA, Lysle DT. Evidence for sympathetic and adrenal involvement in the immunomodulatory effects of acute morphine treatment in rats. J Pharmacol Exp Ther. 1996;277:633–45.
Stefano GB, Hartman A, Bilfinger TV, Magazine HI, Liu Y, et al. Presence of the mu3 opiate receptor in endothelial cells. Coupling to nitric oxide production and vasodilation. J Biol Chem. 1995;270:30290–3.
Fosslien E. Biochemistry of cyclooxygenase (COX)-2 inhibitors and molecular pathology of COX-2 in neoplasia. Crit Rev Clin Lab Sci. 2000;37:431–502.
Locopo N, Fanelli M, Gasparini G. Clinical significance of angiogenic factors in breast cancer. Breast Cancer Res Treat. 1998;52:159–73.
Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–57.
Kim SW, Park SS, Ahn SJ, Chung KW, Moon WK, et al. Identification of angiogenesis in primary breast carcinoma according to the image analysis. Breast Cancer Res Treat. 2002;74:121–9.
Adalet I, Demirkol MO, Müslümanoğlu M, Bozfakioğlu Y, Cantez S. 99Tcm-tetrofosmin scintigraphy in the evaluation of palpable breast masses. Nucl Med Commun. 1997;18:118–21.
Arbab AS, Koizumi K, Toyama K, Araki T. Uptake of technetium-99m-tetrofosmin, technetium-99m-MIBI and thallium-201 in tumor cell lines. J Nucl Med. 1996;37:1551–6.
Taillefer R. The role of 99mTc-sestamibi and other conventional radiopharmaceuticals in breast cancer diagnosis. Semin Nucl Med. 1999;29:16–40.
Kim IJ, Kim SJ, Kim YK. Comparison of double phase Tc-99m MIBI and Tc-99m tetrofosmin scintimammography for characterization of breast lesions: Visual and quantitative analyses. Neoplasma. 2008;55:526–31.
Ohira H, Kubota K, Ohuchi N, Harada Y, Fukuda H, et al. Comparison of intratumoral distribution of 99mTc-MIBI and deoxyglucose in mouse breast cancer models. J Nucl Med. 2000;41:1561–8.
Ballinger JR. 99mTc-tetrofosmin for functional imaging of P-glycoprotein modulation in vivo. J Clin Pharmacol. 2001 Jul;Suppl:39S–47S.
Chen WS, Luker KE, Dahlheimer JL, Pica CM, Luker GD, et al. Effects of MDR1 and MDR3 P-glycoproteins, MRP1, and BCRP/MXR/ABCP on the transport of (99m)Tc-tetrofosmin. Biochem Pharmacol. 2000;60:413–26.
Márián T, Szabó G, Goda K, Nagy H, Szincsák N, et al. In vivo and in vitro multitracer analyses of P-glycoprotein expression-related multidrug resistance. Eur J Nucl Med Mol Imaging. 2003;30:1147–54.
Fox SB, Generali DG, Harris AL. Breast tumour angiogenesis. Breast Cancer Res. 2007;9:216.
Acknowledgment
This study was supported by Trakya University Research Fund (TÜBAP) no. 615.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was presented in part at the Annual Congress of the European Association of Nuclear Medicine-EANM’2008, October 11-15, 2008, Munich, Germany.
Rights and permissions
About this article
Cite this article
Ustun, F., Durmus-Altun, G., Altaner, S. et al. Evaluation of morphine effect on tumour angiogenesis in mouse breast tumour model, EATC. Med Oncol 28, 1264–1272 (2011). https://doi.org/10.1007/s12032-010-9573-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-010-9573-5