Abstract
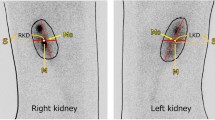
In this study, we investigated the efficacy of 99mTc-DTPA scintigraphic analysis of GFR with the Gates method in comparison with the measurement of plasma urea and creatinine, in the detection of nephrotoxicity occurred in patients treated with cisplatin.Twenty-six male patients with a mean age of 26.73 ± 6.39 years (age range 15–42) who had seminomatous and nonseminomatous testicular carcinoma were included in our study. The patients received cisplatin with a dose of 20 mg/m2 per day for five consecutive days repeated every 21 days. Before starting chemotherapy, immediately after the end of four cycles of chemotherapy and 7 months after the beginning of chemotherapy, plasma urea and creatinine levels were measured and simultaneously scintigraphic GFR estimation using 99 mTc-DTPA with the Gates method was performed. In the measurements done immediately after the chemotherapy, in 18 of the 26 patients GFR levels decreased, in 4 of the 8 remaining patients GFR did not change, and in 4 patients there was an increase in the GFR levels. The changes in the averages of the plasma urea and creatinine levels between measurements done before and after the chemotherapy were not statistically significant. The decrease in the average of the GFR values immediately after chemotherapy, in comparison to the average of GFR values measured before chemotherapy, was found to be statistically significant with paired sample t test analysis (P < 0.009 with 95% CI). We concluded that scintigraphic GFR measurement using the Gates method with 99mTc-DTPA is a suitable method in the diagnosis of nephrotoxicity occuring due to cisplatine.
Similar content being viewed by others
References
Skinner R, et al. Cisplatin dose rate as a risk factor for nephrotoxicity in children. Br J Cancer. 1998;77:1677–82.
Brillet G, et al. Definitive end-stage chronic kidney failure after cisplatin treatment. Nephrologie. 1993;14:227–9.
Cvitkovic E. Cumulative toxicities from cisplatin therapy and current cytoprotective measures. Cancer Treat Rev. 1998;24:265–81.
Daugaard G, et al. Renal tubular function in patients treated with high dose cisplatin. Clin Pharmacol Ther. 1988;44:164–72.
Lam M, Adelstein DJ. Hypomagnesemia and renal magnesium wasting in patients treated with cisplatin. Am J Kidney Dis. 1986;8:164–9.
Guarino AM, et al. Platinate toxicity: past, present and prospect. Cancer Treat Rep. 1979;63:1475–83.
Sugiyama S, et al. Adverse effects of antitumor drug cisplatin on rat kidney mitochondria: disturbance in gluthatione peroxidase activity. Biochem Biophys Res Commun. 1989;59:1121–7.
Bompart G. Cisplatin induced changes in cytochrome platin-450, lipid peroxidation and drug metabolising enzyme activities in rat kidney cortex. Toxicol Lett. 1989;48:193–9.
De Witt LM, Jones TW, Moore L. Stimulation of the renal endoplasmic reticulum calcium pump: a possible biomarker for platinum toxicity. Toxicol Appl Pharmacol. 1988;92:57–69.
Mavichak V, et al. Renal magnesium wasting and hypocalciuria in chronic cisplatin nephropathy in man. Clin Sci. 1988;75:203–7.
Guyton CA. Textbook of medical physiology, Chap. 28. 8th edn. Philadelphia: WB Saunders; 1991. p. 308–329.
Schrier RW. editor. Manual of nephrology, Chap. 14, vol. 5. New York: Little Brown; 1990. p. 285–290.
Dennis VW. Investigation of renal function. In Smith L, editor. Cecil textbook of medicine, Chap. 18, vol. 1. 18th edn. Philadelphia: WB Saunders; 1988. p. 520-8.
Mandal K, Anil Hebert, A Lee, editors. Renal diseases. Med Clinic North Am. 1990;74:859–1077.
Stark JJ, Howell SB. Nephrotoxicity of cisplatin. Clin Pharmacol Ther. 1978;23:461–6.
Brillet G, et al. Long-term renal effect of cisplatin in man. Am J Nephrol. 1994;14(2):81–4.
Schlegel JU, Hamway SA. Individual renal plasma flow determination in 2 minutes. J Urol. 1976;116:282–5.
Gates GF. Glomerular filtration rate: Estimation from fractional accumulation of 99mTc-DTPA. Am J Roentgenol. 1982;3:565–70.
Wagner H, Szabo Z, Buchanan J. Principles of Nuclear Medicine. 2nd ed. Philadelphia: WB Saunders; 1995. p. 966–73.
Petersen PM, Hansen SW. The course of long term toxicity in patients treated with cisplatin based chemotherapy for nonseminomatous germ cell cancer. Ann Oncol. 1999;12:1475–83.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Özülker, F., Özülker, T., Uzun, A.K. et al. Investigation of the efficacy of 99 mTc-DTPA scintigraphic GFR measurement with Gates method in the detection of cisplatin-induced nephrotoxicity in comparison with plasma urea and creatinine measurement. Med Oncol 28, 1101–1106 (2011). https://doi.org/10.1007/s12032-010-9565-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-010-9565-5