Abstract
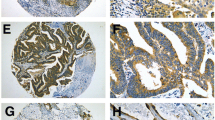
Overall outcome of those patients with non-small cell lung cancer (NSCLC) remains poor. Recently, several studies demonstrated that cyclin-dependent kinase-5 (CDK5) activity with its specific activator protein p35 was important for spontaneous metastasis in various types of carcinomas. Our objective was to explore the expression of CDK5 and its prognostic indicator in patients with NSCLC. Immunofluorescent staining was used to detect the expression of CDK5/p35 in the lung tissue of 95 patients with NSCLC and 20 patients with benign pulmonary disease. The correlation between the expression of CDK5/p35 and clinicopathologic features of patients with NSCLC was investigated. The 5-year overall survival of patients with tumors expressing different levels of CDK5/p35 was evaluated by the Kaplan–Meier method. Positive expressions of CDK5/p35 were detected in the tumor cells in 66 samples (69.5%) of the 95 patients with NSCLC. Although no remarkable correlation between CDK5/p35 expression and age at the time of surgery, gender, and histopathologic type, there were significant differences between CDK5/p35 expression and degree of differentiation, pathological stage and lymph node metastasis in patients with NSCLC. In addition, we demonstrated that median survival for patients with and without CDK5/p35 expression was 24 and 58 months, respectively, and 5-year overall survival rate 25.8 and 48.3%, respectively (P < 0.05). Patients with lung cancer with a positive CDK5/p35 expression had a poorer prognosis than those with a negative CDK5/p35 expression. Based on our results, CDK5/p35 may represent a biomarker for prognosis in patients with NSCLC.


Similar content being viewed by others
References
Pirozynski M. 100 years of lung cancer. Respir Med. 2006;100:2073–84.
Baggstrom MQ, Stinchcombe TE, Fried DB, Poole C, Hensing TA, Socinski MA. Third-generation chemotherapy agents in the treatment of advanced non-small cell lung cancer: a meta-analysis. J Thorac Oncol. 2007;2:845–53.
Non-small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ. 1995;311:899–909.
Tsai LH. A Jekyll and Hyde kinase: roles for Cdk5 in brain development and disease. Curr Opin Neurobiol. 2004;14:390–4.
Gao C, Negash S, Wang HS, Ledee D, Guo H, Russell P, et al. Cdk5 mediates changes in morphology and promotes apoptosis of astrocytoma cells in response to heat shock. J Cell Sci. 2001;114:1145–53.
Sandal T, Stapnes C, Kleivdal H, Hedin L, Doskeland SO. A novel, extraneuronal role for cyclin-dependent protein kinase 5 (CDK5): modulation of cAMP-induced apoptosis in rat leukemia cells. J Biol Chem. 2002;277:20783–93.
Strock CJ, Park JI, Nakakura EK, Bova GS, Isaacs JT, Ball DW, et al. Cyclin-dependent kinase 5 activity controls cell motility and metastatic potential of prostate cancer cells. Cancer Res. 2006;66:7509–15.
Goodyear S, Sharma MC. Roscovitine regulates invasive breast cancer cell (MDA-MB231) proliferation and survival through cell cycle regulatory protein cdk5. Exp Mol Pathol. 2007;82:25–32.
Lin H, Chen MC, Chiu CY, Song YM, Lin SY. Cdk5 regulates STAT3 activation and cell proliferation in medullary thyroid carcinoma cells. J Biol Chem. 2007;282:2776–84.
Sahai E. Mechanisms of cancer cell invasion. Curr Opin Genet Dev. 2005;15:87–96.
Ridley AJ. Rho proteins and cancer. Breast Cancer Res Treat. 2004;84:13–9.
Bielas SL, Gleeson JG. Cytoskeletal-associated proteins in the migration of cortical neurons. J Neurobiol. 2004;58:149–59.
Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–9.
Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–79.
Wang W, Goswami S, Sahai E, Wyckoff JB, Segall JE, Condeelis JS. Tumor cells caught in the act of invading: their strategy for enhanced cell motility. Trends Cell Biol. 2005;15:138–45.
Wang W, Goswami S, Lapidus K, Wells AL, Wyckoff JB, Sahai E, et al. Identification and testing of a gene expression signature of invasive carcinoma cells within primary mammary tumors. Cancer Res. 2004;64:8585–94.
Lew J, Huang QQ, Qi Z, Winkfein RJ, Aebersold R, Hunt T, et al. A brain-specific activator of cyclin-dependent kinase 5. Nature. 1994;371:423–6.
Tsai LH, Delalle I, Caviness VS Jr, Chae T, Harlow E. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature. 1994;371:419–23.
Lockwood WW, Chari R, Coe BP, Girard L, Macaulay C, Lam S, et al. DNA amplification is a ubiquitous mechanism of oncogene activation in lung and other cancers. Oncogene. 2008;27:4615–24.
Choi HS, Lee Y, Park KH, Sung JS, Lee JE, Shin ES, et al. Single-nucleotide polymorphisms in the promoter of the CDK5 gene and lung cancer risk in a Korean population. J Hum Genet. 2009;54:298–303.
Schutte B, Nieland L, van Engeland M, Henfling ME, Meijer L, Ramaekers FC. The effect of the cyclin-dependent kinase inhibitor olomoucine on cell cycle kinetics. Exp Cell Res. 1997;236:4–15.
Garrett MD, Fattaey A. CDK inhibition and cancer therapy. Curr Opin Genet Dev. 1999;9:104–11.
Acknowledgments
The investigation was supported by a grant of National Natural Science Foundation of China (30973473 to professor Gang Wu).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, JL., Wang, XY., Huang, BX. et al. Expression of CDK5/p35 in resected patients with non-small cell lung cancer: relation to prognosis. Med Oncol 28, 673–678 (2011). https://doi.org/10.1007/s12032-010-9510-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-010-9510-7