Abstract
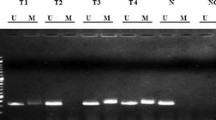
PTEN/MMAC1/TEP1 encodes a tumor suppressor protein, which regulates cell cycle progression, translation, and apoptosis by blocking the activation of Akt/PKB. The loss of PTEN function increases cell survival and induces tumor invasion. In this study, PTEN promoter status and its correlation with genetic and pathologic parameters were analyzed in genomic DNA from Iranian patients with breast cancer. DNA methylation patterns in the CpG islands were determined by a methylation-specific PCR (MSP) assay. PTEN promoter methylation was found to be present in 37 of 53(70%) tumor tissues and none in 20 normal counterparts. Moreover, promoter methylation was found in patients with heterozygote mutation in the PTEN gene. The pathological history of cancerous tissue sections showed that PTEN gene could be inactivated at the stages III and IV in sporadic breast cancer. These findings suggested that promoter hypermethylation of PTEN might contribute to the progression of sporadic breast cancer in human.


Similar content being viewed by others
References
Stambolic V, Suzuki A, Pompa JL, Brothers GM, Mirtsos C, Sasaki T, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39.
Gu J, Tamura M, Pankov R, Danen EH, Takino T, Matsumoto K, et al. Shc and FAK differentially regulate cell motility and directionality modulated by PTEN. J Cell Biol. 1999;146:389–403.
Freeman DJ, Li AG, Wei G, Li H-H, Kertesz N, Lesche R, et al. PTEN tumor suppressor regulates p53 protein levels and activity through phosphatase-dependent and -independent mechanisms. Cancer Cell. 2003;3:117–30.
Li AG, Piluso LG, Cai X, Wei G, Sellers WR, Liu X. Mechanistic insights into maintenance of high p53 acetylation by PTEN. Mol Cell. 2006;23:575–87.
Reifenberger J, Wolter M, Bostrom J, et al. Allelic losses on chromosome arm 10q and mutation of the PTEN (MMAC1) tumour suppressor gene in primary and metastatic malignant melanomas. Virchows Arch. 2000;436:487–93.
Piekarski JH, Biernat W. Clinical significance of CK5/6 and PTEN protein expression in patients with bilateral breast carcinoma. Histopathology. 2006;49:248–55.
Li Z, Wings TY, Chow LWC. PTEN and VEGF: possible predictors for sentinel lymph node micro-metastasis in breast cancer. Biomed Pharmacother. 2007;61:558–61.
Mise-Omataa S, Obataa Y, Iwaseb S, Misec N, Doia T. Transient strong reduction of PTEN expression by specific RNAi induces loss of adhesion of the cells. Biochem Biophys Res. 2005;38:1034–42.
Stambolic V, Tsao MS, Macpherson D, Suzuki A, Chapman WB, Mak TW. High incidence of breast and endometrial neoplasia resembling human Cowden syndrome in pten±mice. Cancer Res. 2000;60(13):3605–11.
Nassiri I, Tavasoli M, Faghihi M. Pharmacogenomic profiling of the PI3 K/PTEN pathway in sporadic breast cancer. Iran Biomed J. 2008;12(4):209–14.
Nassiri I, Tavasoli M, Faghihi M. Mutational analysis in the MMAC1 gene associated in patients with juvenile polyposis syndrome and cowden fisease. Austral Asian J Can. 2008;7(3):25–30.
Sehgal SN, Baker H, Vezina C. Rapamycin (AY-22, 989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J Antibiot. 1975;28:727–32.
Singer C, Köstler W, Hudelist G. Predicting the efficacy of trastuzumab-based therapy in breast cancer: Current standards and future strategies. Biochim Biophys Acta. 2008;1786(2):105–13.
Sambrook J, Fritsh EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 2001.
Miller SA, Dykes DD, Polesky HF. A simple salting-out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215.
Zysman MA, Chapman WB, Bharati B. Considerations when analyzing the methylation status of PTEN tumor suppressor gene. Am J Pathol. 2002;160:795–800.
Baker SJ. PTEN enters the nuclear age. Cell. 2007;128:25–8.
Bose S, Crane A, Hibshoosh H, Mansukhani M, Sandweis L, Parsons R. Reduced expression of PTEN correlates with breast cancer progression. Hum Pathol. 2002;33:405–9.
Khan K, Umagal K, Vora J, Bose N, Sehgal S, Koeffler E, et al. PTEN promoter is methylated in a proportion of invasive breast cancer. Int J Cancer. 2004;112:407–10.
Kang Y, Lee HS, Ho KW. Promoter methylation and silencing of PTEN in gastric carcinoma. Lab Invest. 2002;82:285–91.
Chang S, Lee SN, Cho MS, Koo H, Han WS, Im SA, et al. Loss of PTEN expression in breast cancers. Korean J Path. 2005;39:236–41.
Acknowledgments
The authors wish to thank Dr M Faghihi from breast cancer section of the Seyed Al-Shohada Hospital, Isfahan, IR, for providing the normal and breast carcinoma tissues. This work was supported through a general grant (Pajoohaneh) by the department of research of University of Isfahan to S Vallian.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sadeq, V., Isar, N. & Manoochehr, T. Association of sporadic breast cancer with PTEN/MMAC1/TEP1 promoter hypermethylation. Med Oncol 28, 420–423 (2011). https://doi.org/10.1007/s12032-010-9473-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-010-9473-8