Abstract
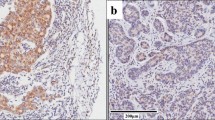
Esophageal squamous cell carcinoma (ESCC) is one of the most frequently diagnosed malignant tumors in North China. We have identified that Wnt2/β-catenin pathway is activated in ESCC cells and sodium nitroprusside (SNP) and siRNA against β-catenin not only inhibit the expressions of β-catenin and its major downstream effectors including c-myc and cyclin D1 but induce cell cycle arrest and apoptosis. The purpose of the present study was to analyze the relationship between pathological parameters including invasion depth and lymph node metastasis and the expressions of β-catenin, c-myc, and cyclin D1 in order to evaluate their values of prognosis in patients with ESCC. The expressions of β-catenin, c-myc, and cyclin D1 were detected immunohistochemically in the resected cancer tissues from 40 patients with ESCC. The β-catenin expression was reduced in 22 (55.0%) patients, which was closely correlated with invasion depth (P = 0.023) and lymph node metastasis (P = 0.003). There was the positive c-myc expression in 21 (52.5%), which was significantly correlated with invasion depth (P = 0.009) and lymph node metastasis (P = 0.001). Furthermore, the results of survival rates analyzed by Kaplan–Meier curve revealed that patients with the reduced expression of β-catenin had a poorer prognosis than those with the preserved expression (P = 0.031), and patients with the positive expression of c-myc also had a significantly poorer prognosis than those with the negative expression (P = 0.008). These findings demonstrate that β-catenin pathway plays a crucial role in the progression of ESCC, suggesting that both β-catenin and c-myc may be used as markers for predicting the prognosis of patients with ESCC.


Similar content being viewed by others
References
Pisani P, Parkin DM, Bray F, et al. Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer. 1999;83:18–29.
Montesano R, Hollstein M, Hainaut P. Genetic alterations in esophageal cancer and their relevance to etiology and pathogenesis: a review. Int J Cancer. 1996;69:225–35.
Conacci-Sorrell M, Zhurinsky J, Ben-Ze’ev A. The cadherin-catenin adhesion system in signaling and cancer. J Clin Invest. 2002;109:987–91.
Day TF, Yang Y. Wnt and Hedgehog signaling pathways in bone development. J Bone Joint Surg. 2008;90:19–24.
Muzio L. A possible role for the Wnt-1 pathway in oral carcinogenesis. Crit Rev Oral Biol Med. 2001;12:152–65.
Holcombe RF, Marsh JL, Waterman ML, et al. Expression of Wnt ligands and Frizzled receptors in colonic mucosa and in colon carcinoma. Mol Pathol. 2002;55:220–6.
Lustig B, Behrens J. The Wnt signaling pathway and its roles in tumor development. J Cancer Res Clin Oncol. 2003;129:199–221.
Krieghoff E, Behrens J, Mayr B. Nucleo-cytoplasmic distribution of β-catenin is regulated by retention. J Cell Sci. 2006;119:1453–63.
Willert K, Jones KA. Wnt signaling: is the party in the nucleus? Genes Dev. 2006;20:1394–404.
Barbara G, De Giorgio R, Stanghellini V, et al. Neutral endopeptidase (EC 3.4.24.11) downregulates the onset of intestinal inflammation in the nematode infected mouse. Gut. 2003;52:1457–64.
Poston RS Jr, Billingham ME, et al. Effects of increased ICAM-1 on reperfusion injury and chronic graft vascular disease. Ann Thorac Surg. 1997;64:1004–12.
Hou G, Xue L, Lu Z, et al. An activated mTOR/p70S6 K signaling pathway in esophageal squamous cell carcinoma cell lines and inhibition of the pathway by rapamycin and siRNA against mTOR. Cancer Lett. 2007;253:236–48.
Kase S, Sugio K, Yamazaki K, et al. Expression of E-cadherin and β-catenin in human non-small cell lung cancer and the clinical significance. Clin Cancer Res. 2000;6:4789–96.
Tang S, Chang W, Su Y, et al. Myc pathway is activated in clear cell renal cell carcinoma and essential for proliferation of clear cell renal cell carcinoma cells. Cancer Lett. 2009;273:35–43.
Bani-Hani K, Martin IG, Hardie LJ, et al. Prospective study of cyclin D1 overexpression in Barrett’s esophagus: association with increased risk of adenocarcinoma. J Natl Cancer Inst. 2000;92:1316–21.
Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–52.
Tachibana M, Kinugasa S, Yoshimura H, et al. Clinical outcomes of extended esophagectomy with three-field lymph node dissection for esophageal squamous cell carcinoma. Am J Surg. 2005;189:98–109.
Conroy T, Etienne PL, Adenis A, et al. Vinorelbine and cisplatin in metastatic squamous cell carcinoma of the esophagus: response, toxicity, quality of life and survival. Ann Oncol. 2002;13:721–9.
Nair KS, Naidoo R, Chetty R. Expression of cell adhesion molecules in esophageal carcinoma and its prognostic value. J Clin Pathol. 2005;58:343–51.
Deng YZ, Chen PP, Wang Y, et al. Connective tissue growth factor is overexpressed in esophageal squamous cell carcinoma and promotes tumorigenicity through β-catenin-T-cell factor/lef signaling. J Biol Chem. 2007;282:36571–81.
Hsu P, Li A, Wang Y, et al. Reduced membranous β-catenin protein expression is associated with metastasis and poor prognosis in squamous cell carcinoma of the esophagus. J Thorac Cardiovasc Surg. 2008;135:1029–35.
Retera JM, Leers MP, Sulzer MA, et al. The expression of β-catenin in non-small-cell lung cancer: a clinicopathological study. J Clin Pathol. 1998;51:891–4.
Dvory-Sobol H, Sagiv E, Kazanov D, et al. Targeting the active ß-catenin pathway to treat cancer cells. Mol Cancer Ther. 2006;5:2861–71.
Calvo R, Drabkin HA. Embryonic genes in cancer. Ann Oncol. 2000;11:207–18.
Uraguchi M, Morikawa M, Shirakawa M, et al. Activation of Wnt family expression and signaling in squamous cell carcinomas of the oral cavity. J Dent Res. 2004;83:327–32.
Nakopoulou L, Mylona E, Papadaki I, et al. Study of phospho-β-catenin subcellular distribution in invasive breast carcinomas in relation to their phenotype and the clinical outcome. Mod Pathol. 2006;19:556–63.
Veeramachaneni NK, Kubokura H, Lin L, et al. Down-regulation of β-catenin inhibits the growth of esophageal carcinoma cells. J Thorac Cardiovasc Surg. 2004;127:92–8.
You Z, Saims D, Chen S, et al. Wnt signaling promotes oncogenic transformation by inhibiting c-myc–induced apoptosis. J Cell Biol. 2002;157:429–40.
Cowling VH, D’Cruz CM, Chodosh LA, et al. c-myc transforms human mammary epithelial cells through repression of the Wnt inhibitors DKK1 and SFRP1. Mol Cell Biol. 2007;27:5135–46.
Muncan V, Sansom OJ, Tertoolen L, et al. Rapid loss of intestinal crypts upon conditional deletion of the Wnt/Tcf4 target gene c-myc. Mol Cell Biol. 2006;26:8418–26.
Wolter F, Akoglu B, Clausnitzer A, et al. Down regulation of the cyclin D1/CDK4 complex occurs during resveratrol induced cell cycle arrest in colon cancer cell lines. J Nutr. 2001;131:2197–203.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, W., Xue, L. & Wang, P. Prognostic value of β-catenin, c-myc, and cyclin D1 expressions in patients with esophageal squamous cell carcinoma. Med Oncol 28, 163–169 (2011). https://doi.org/10.1007/s12032-010-9436-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-010-9436-0