Abstract
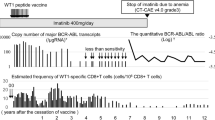
Although antigen-specific immune responses including cytotoxic T cells (CTLs) against antigen peptide could be enhanced after tumor antigen peptide vaccinations, the immune responses do not necessarily result in a decrease or eradication of tumor cells in the vaccination trials. We focused on whether antigen-specific CTLs could be damaged by the repeated stimulation of antigenic peptide and whether regulatory T (Treg) cells would be increased by the administration of WT1 peptide. We administered WT1 peptide 22 times over 18 months in a CML patient who was being treated with imatinib. Although WT1 peptide administration every 2 weeks did not show any beneficial effects on the minimal residual disease (copies of bcr-abl transcripts), the transcripts remarkably decreased to the level of major molecular response after changing the administration interval of WT1 peptide from 2 to 4 weeks. An ex vivo study demonstrated that re-stimulation with WT1 peptide made WT1-specific T cells less reactive to WT1 tetramers and the impaired reactivity of CTLs lasted at least for 1 week. In addition, the cytotoxicity of the T cells was hampered by re-stimulation. Treg cells increased up to more than fivefold at the end of the WT1 administration period. The present findings suggested that the administration of the peptide every 4 weeks is superior to every 2 weeks. In addition, the findings that Treg cells increased gradually in accordance with the duration of WT1 peptide administration revealed the significance of manipulating Treg cells for establishing an efficient tumor antigen peptide vaccination.









Similar content being viewed by others
References
Dauer M, Schnurr M, Eigler A. Dendritic cell-based cancer vaccination: quo vadis? Expert Rev Vaccines. 2008;7:1041–53.
Oka Y, Tsuboi A, Oji Y, Kawase I, Sugiyama H. WT1 peptide vaccine for the treatment of cancer. Curr Opin Immunol. 2008;20:211–20.
Petrausch U, et al. Cancer immunotherapy: the role regulatory T cells play and what can be done to overcome their inhibitory effects. Curr Mol Med. 2009;9:673–82.
Demotte N, et al. Restoring the association of the T cell receptor with CD8 reverses anergy in human tumor-infiltrating lymphocytes. Immunity. 2008;28:414–24.
Oka Y, et al. Development of WT1 peptide cancer vaccine against hematopoietic malignancies and solid cancers. Curr Med Chem. 2006;13:2345–52.
Izumoto S, et al. Phase II clinical trial of Wilms tumor 1 peptide vaccination for patients with recurrent glioblastoma multiforme. J Neurosurg. 2008;108:963–71.
Oka Y, et al. Induction of WT1 (Wilms’ tumor gene)-specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression. Proc Natl Acad Sci U S A. 2004;101:13885–90.
Tsuboi A, et al. WT1 peptide-based immunotherapy for patients with lung cancer: report of two cases. Microbiol Immunol. 2004;48:175–84.
Iiyama T, et al. WT1 (Wilms’ tumor 1) peptide immunotherapy for renal cell carcinoma. Microbiol Immunol. 2007;51:519–30.
Ohta H, et al. WT1 (Wilms tumor 1) peptide immunotherapy for childhood rhabdomyosarcoma: a case report. Pediatr Hematol Oncol. 2009;26:74–83.
Yasukawa M, et al. Clinical efficacy of WT1 peptide vaccination in patients with acute myelogenous leukemia and myelodysplastic syndrome. Am J Hematol. 2009;84:314–5.
Mailander V, et al. Complete remission in a patient with recurrent acute myeloid leukemia induced by vaccination with WT1 peptide in the absence of hematological or renal toxicity. Leukemia. 2004;18:165–6.
Rezvani K, et al. Leukemia-associated antigen-specific T-cell responses following combined PR1 and WT1 peptide vaccination in patients with myeloid malignancies. Blood. 2008;111:236–42.
Keilholz U, et al. A clinical and immunologic phase 2 trial of Wilms tumor gene product 1 (WT1) peptide vaccination in patients with AML and MDS. Blood. 2009;113:6541–8.
Kawakami M, et al. Clinical and immunologic responses to very low-dose vaccination with WT1 peptide (5 microg/body) in a patient with chronic myelomonocytic leukemia. Int J Hematol. 2007;85:426–9.
Tsuboi A, et al. Wilms tumor gene WT1 peptide-based immunotherapy induced a minimal response in a patient with advanced therapy-resistant multiple myeloma. Int J Hematol. 2007;86:414–7.
Oka Y, et al. Wilms tumor gene peptide-based immunotherapy for patients with overt leukemia from myelodysplastic syndrome (MDS) or MDS with myelofibrosis. Int J Hematol. 2003;78:56–61.
Tsuboi A, et al. Enhanced induction of human WT1-specific cytotoxic T lymphocytes with a 9-mer WT1 peptide modified at HLA-A*2402-binding residues. Cancer Immunol Immunother. 2002;51:614–20.
Karanikas V, et al. Monoclonal anti-MAGE-3 CTL responses in melanoma patients displaying tumor regression after vaccination with a recombinant canarypox virus. J Immunol. 2003;171:4898–904.
Furukawa T, et al. Establishment of a new cell line with the characteristics of a multipotential progenitor from a patient with chronic myelogenous leukemia in early erythroblastic crisis. Leukemia. 1994;8:171–80.
Beyer M, Schultze JL. Regulatory T cells in cancer. Blood. 2006;108:804–11.
Dannull J, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–33.
Wolf AM, et al. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606–12.
Merlo A, et al. FOXP3 expression and overall survival in breast cancer. J Clin Oncol. 2009;27:1746–52.
Chappert P, et al. Antigen-specific Tregs impair CD8 T cell priming by blocking early T cell expansion. Eur J Immunol. 2009.
Demotte N, et al. A reversible functional defect of CD8 + T lymphocytes involving loss of tetramer labeling. Eur J Immunol. 2002;32:1688–97.
Lin RS, Rodriguez C, Veillette A, Lodish HF. Zinc is essential for binding of p56(lck) to CD4 and CD8alpha. J Biol Chem. 1998;273:32878–82.
Lissina A, et al. Protein kinase inhibitors substantially improve the physical detection of T-cells with peptide-MHC tetramers. J Immunol Methods. 2009;340:11–24.
Morgan R, et al. N-acetylglucosaminyltransferase V (Mgat5)-mediated N-glycosylation negatively regulates Th1 cytokine production by T cells. J Immunol. 2004;173:7200–8.
Acknowledgments
We appreciate Drs. Manabu Kawakami, Yoshihiro Oka and Haruo Sugiyama (Department of Functional Diagnostic Science, Osaka University Graduate School of Medicine, Osaka, Japan) for designing the protocol of the WT1 peptide vaccinations and giving us valuable advices for the study, and Drs. Shoko Takenouchi, Toshiki Kitajima and Tohri Ida (Division of Hematology, Graduate School of Medical and Dental Sciences, Niigata University, Niigata, Japan) for treating the CML patients with WT1 peptide vaccination. We appreciate Dr. Susumu Suzuki (T cell Technology, Ina, Japan) for his generous advice in this study. We thank Dr. Kiyotaka Kuzushima (Aichi Cancer Center Research Institute, Nagoya, Japan) for kindly donating modified-type WT1 tetramers and HIV env tetramers and Dr. Shingo Toji (MBL, Ina, Nagano, Japan) for kindly donating FITC-labeled modified-type WT1 tetramers.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saitoh, A., Narita, M., Watanabe, N. et al. WT1 peptide vaccination in a CML patient: induction of effective cytotoxic T lymphocytes and significance of peptide administration interval. Med Oncol 28, 219–230 (2011). https://doi.org/10.1007/s12032-010-9425-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-010-9425-3