Abstract
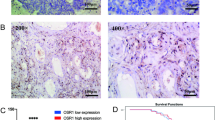
Ovarian cancer is a leading cause of cancer-related women mortality in China. In recent years, the molecular mechanisms involved in ovarian carcinoma development and/or progression have been intensely studied, and several genes have been identified. Deleted in Colorectal Carcinoma (DCC), is an important tumor suppressor gene, which is inactivated in many kinds of tumors, and its function(s) is not clarified. Even though the lost expression of DCC occurred in later stages of multistep colorectal carcinogenesis, its contribution to the onset or progression of ovarian cancer is not fully understood. To investigate DCC expression in ovarian cancer, we studied 254 clinical samples by RT–PCR. Our results revealed that 52% malignant ovarian cancer did not express DCC gene. By contrast, DCC expression was observed in all normal ovary tissues and 80% benign ovarian tumors. Obviously, there was a significant correlation between DCC expression and ovarian cancer, especially in the epithelial ovarian cancer. The present study also suggested that the loss expression of DCC occurred more frequently in the cases of later clinical stage, higher pathological grade, and poorer prognosis. In the other part of this study, we further explored DCC expression after transfection in two kinds of ovarian caner cell lines, namely SKOV3 cell and HO-8910 cell, using RT–PCR and immunocytochemistry. The results indicated that DCC expressed in SKOV3-DCC and HO-8910-DCC cells, and ultrastructural analysis showed the appearance of apoptotic features in them. Furthermore, cell growth was markedly down-regulated in above groups of cells, indicating that transfection with the DCC constructs can suppress the growth of tumor cells. In conclusion, our results suggest an association of lost expression of DCC with the ovarian cancer, and DCC gene may inhibit the growth of ovarian carcinoma cells. However, this result needs further trials with a larger sample.






Similar content being viewed by others
References
Bamias A, Yu Z, Weinberger PM, et al. Automated quantitative analysis of DCC tumor suppressor protein in ovarian cancer tissue microarray shows association with beta-catenin levels and outcome in patients with epithelial ovarian cancer. Ann Oncol. 2006;17(12):1797–802.
Yan L, Na W, Shan K, et al. p16(CDKN2) gene polymorphism: association with histologic subtypes of epithelial ovarian cancer in China. Int J Gynecol Cancer. 2008;18(1):30–5.
Yang XY, Yu H, Xi MR, et al. Association of the ARLTS1 variants with familial ovarian cancer risk in China. Int J Gynecol Cancer. 2009;19(4):585–90.
Wan T, Liu JH, Zheng LM, et al. Prognostic significance of tumor-associated macrophage infiltration in advanced epithelial ovarian carcinoma. Ai Zheng. 2009;28(3):323–7.
Murph MM, Liu W, Yu S, et al. Lysophosphatidic acid-induced transcriptional profile represents serous epithelial ovarian carcinoma and worsened prognosis. PLoS One. 2009;4(5):e5583.
Rodrigues S, De Wever O, Bruyneel E, et al. Opposing roles of netrin-1 and the dependence receptor DCC in cancer cell invasion, tumor growth and metastasi. Oncogene. 2007;26(38):5615–25.
Martín M, Simon-Assmann P, Kedinger M, et al. DCC regulates cell adhesion in human colon cancer derived HT-29 cells and associates with ezrin. Eur J Cell Biol. 2006;85(8):769–83.
Shin SK, Nagasaka T, Jung BH, et al. Epigenetic and genetic alterations in Netrin-1 receptors UNC5C and DCC in human colon cancer. Gastroenterology. 2007;133(6):1849–57.
Derks S, Bosch LJ, Niessen HE, et al. Promoter CpG island hypermethylation- and H3K9me3 and H3K27me3-mediated epigenetic silencing targets the deleted in colon cancer (DCC) gene in colorectal carcinogenesis without affecting neighboring genes on chromosomal region 18q21. Carcinogenesis. 2009;30(6):1041–8.
Wu JT, Kakar S, Nelson RL, et al. Prognostic significance of DCC and p27Kip1 in colorectal cancer. Appl Immunohistochem Mol Morphol. 2005;13(1):45–54.
Klingelhutz AJ, Hedrick L, Cho KR, et al. The DCC gene suppresses the malignant phenotype of transformed human epithelial cells. Oncogene. 1995;10:1581–6.
Fujita M, Enomoto T, Murata Y. Genetic alterations in ovarian carcinoma: with specific reference to histological subtypes. Mol Cell Endocrinol. 2003;202:97–9.
Fearon ER, Cho KR, Nigro JM, et al. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990;247:49–56.
Tanaka K, Oshimura M, Kikuchi R, et al. Suppression of tumorigenicity in human colon carcinoma cells by introduction of normal chromosome 5 or 18. Nature. 1991;349:340–2.
Saegusa M, Machida D, Okayasu I. Loss of DCC gene expression during ovarian tumorigenesis: relation to tumour differentiation and progression. Br J Cancer. 2000;82:571–8.
Liu J, Yao F, Wu R, et al. Mediation of the DCC apoptotic signal by DIP13 alpha. J Biol Chem. 2002;277:26281–5.
Takakura S, Okamoto A, Saito M, et al. Allelic imbalance in chromosome band 18q21 and SMAD4 mutations in ovarian cancers. Genes Chromosomes Cancer. 1999;24:264–71.
Chenevix-Trench G, Leary J, Kerr J, et al. Frequent loss of heterozygosity on chromosome 18 in ovarian adenocarinoma which does not always include the DCC locus. Oncogene. 1992;7:1059–65.
Lassus H, Salovaara R, Aaltonen LA, et al. Allelic analysis of serous ovarian carcinoma reveals two putative tumor suppressor loci at 18q22–q23 distal to SMAD4, SMAD2, and DCC. Am J Pathol. 2001;159:35–42.
Forcet C, Ye X, Granger L, et al. The dependence receptor DCC (deleted in colorectal cancer) defines an alternative mechanism for caspase activation. Proc Natl Acad Sci USA. 2001;98:3416–21.
Schwartz DR, Kardia SLR, Shedden KA, et al. Gene expression in ovarian cancer reflects both morphology and biological behavior, distinguishing clear cell from other poor-prognosis ovarian carcinomas. Cancer Res. 2002;62:4722–9.
Jen J, Kim H, Piantadosi S, et al. Allelic loss of chromosome 18q and prognosis in colorectal cancer. N Engl J Med. 1994;331:213–21.
Inoue T, Uchino S, Shiraishi N, et al. Loss of heterozygosity on chromosome 18q in cohesive-type gastric cancer is associated with tumor progression and poor prognosis. Clin Cancer Res. 1998;4:973–7.
Tapper J, Sarantaus L, Vahteristo P, et al. Genetic changes in inherited and sporadic ovarian carcinomas by comparative genomic hybridization: extensive similarity except for a difference at chromosome 2q24–q32. Cancer Res. 1998;58:2715–9.
Pere H, Tapper J, Seppala M, et al. Genomic alterations in fallopian tube carcinomas: comparison to serous uterine and ovarian carcinomas reveals similarity suggesting likeness in molecular pathogenesis. Cancer Res. 1998;58:4274–6.
Liu JR, Opipari AW, Tan L, et al. Dysfunctional apoptosome activation in ovarian cancer: implications for chemoresistance. Cancer Res. 2002;62:924–31.
Acknowledgments
We thank Prof Li Baoxin of Harbin Medical University for her advice on our study and statistical analysis. This project was supported by a grant from the Provincial Government Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dr Liu Meimei now works as a post-doctoral in College of Pharmacy, Harbin Medical University.
Rights and permissions
About this article
Cite this article
Meimei, L., Peiling, L., Baoxin, L. et al. Lost expression of DCC gene in ovarian cancer and its inhibition in ovarian cancer cells. Med Oncol 28, 282–289 (2011). https://doi.org/10.1007/s12032-009-9400-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-009-9400-z