Abstract
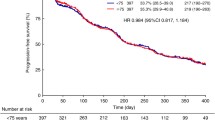
Renal cell carcinoma primarily affects older individuals. Approximately half of all new renal cell carcinoma diagnoses are made in persons 65 years of age or older. Devising a treatment plan for the elderly patient population requires special consideration. Age-related physiological, cognitive, and social characteristics of elderly patients may influence each stage of patient care. Until recently, treatment options were limited for elderly patients with renal cell carcinoma. Sorafenib is the first multikinase inhibitor approved for use in renal cell carcinoma in the United States and Europe. In the phase III Treatment Approaches in Renal Cell Cancer Global Evaluation Trial, sorafenib significantly extended progression-free survival in patients with advanced renal cell carcinoma, regardless of age. Incidence rates of adverse events were not significantly higher in elderly patients receiving sorafenib than in younger patients. Thus, sorafenib represents an important treatment option for elderly patients with renal cell carcinoma. This report describes particular considerations for physicians to be aware of when choosing a treatment regimen for their elderly patients with renal cell carcinoma and offers recommendations on how to integrate specific management strategies into clinical practice that will optimize the use of sorafenib in the elderly. The strategies focus on patient selection, assessment of quality of life, management of adverse events, and appropriate dose modifications. The goal of these recommendations is to maximize the clinical benefit of sorafenib in the elderly patient population through appropriate use.


Similar content being viewed by others
References
Wilkins M. Cancer in the elderly patient. In: Pathy MSJ, Sinclair AJ, Morley JE, editors. Principles and Practices of Geriatric Medicine. Chichester: Wiley; 1991. p. 1385–96.
National Cancer Institute SEER Database. Cancer: renal and kidney pelvis. 2009. Available at: http://www.seer.cancer.gov./statfacts/html/kidrp.html. Accessed 16 Feb 2009.
Martin JE, Sheaff MT. The pathology of ageing: concepts and mechanisms. J Pathol. 2007;211:111–3.
Bellmunt J, et al. The medical treatment of metastatic renal cell cancer in the elderly: position paper of a SIOG Taskforce. Crit Rev Oncol Hematol. 2009;69:64–72.
Coebergh JW, Janssen-Heijnen ML, Post PN, Razenberg PP. Serious co-morbidity among unselected cancer patients newly diagnosed in the southeastern part of The Netherlands in 1993–1996. J Clin Epidemiol. 1999;52:1131–6.
Extermann M. Interaction between comorbidity and cancer. Cancer Control. 2007;14:13–22.
Taccoen X, et al. Renal cell carcinoma in adults 40 years old or less: young age is an independent prognostic factor for cancer-specific survival. Eur Urol. 2007;51:980–7.
National Cancer Institute. FDA approval for Sorafenib Tosylate. National Cancer Institute. 2007. Available at: http://www.cancer.gov/cancertopics/druginfo/fda-sorafenib-tosylate#Anchor-Kidne-39939. Accessed 7 July 2009.
European Medicines Agency. Public summary of positive opinion for orphan designation of sorafenib tosylate for the treatment of renal cell carcinoma. 2009. Available at: http://www.emea.europa.eu/pdfs/human/comp/opinion/029404en.pdf. Accessed 10 Feb 2009.
Wilhelm SM, et al. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–40.
Escudier B, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–34.
Escudier B, et al. Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase III Treatment Approaches in Renal Cancer Global Evaluation Trial. J Clin Oncol. 2009;27(20):3312–8.
Stadler W, et al. Safety and efficacy results of the advanced renal cell carcinoma sorafenib (ARCCS) expanded access program in North America. Cancer. 2009. (in press).
Beck J, et al. A large open-label, non-comparative, phase III study of the multi-targeted kinase inhibitor sorafenib in European patients with advanced renal cell carcinoma. Presented at ECCO 14, The European Cancer Conference, Barcelona, Spain; 23–27 September 2007. Abstract 4506.
Rosenberg SA, et al. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg. 1989;210:474–84.
Eisen T, et al. Sorafenib for older patients with renal cell carcinoma: subset analysis from a randomized trial. J Natl Cancer Inst. 2008;100:1454–63.
Porta C, et al. Efficacy and safety of sorafenib in elderly patients: results from a large open-label, non-comparative phase III study in European patients with advanced RCC (EU-ARCCS). Ann Oncol. 2008;19(suppl 8):viii193.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: kidney cancer. 2009. Available at: http://www.nccn.org/professionals/physician_gls/PDF/kidney.pdf. Accessed 26 Mar 2009.
Droz JP, Chaladaj A. Management of metastatic prostate cancer: the crucial role of geriatric assessment. BJU Int. 2008;101(suppl 2):23–9.
Bulpitt CJ, et al. The assessment of biological age: a report from the Department of Environment Study. Aging (Milano). 1994;6:181–91.
Aapro MS. The frail are not always elderly. J Clin Oncol. 2005;23:2121–2.
Walter LC, et al. Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA. 2001;285:2987–94.
Maas HA, Janssen-Heijnen ML, Olde Rikkert MG, Machteld Wymenga AN. Comprehensive geriatric assessment and its clinical impact in oncology. Eur J Cancer. 2007;43:2161–9.
Passage KJ, McCarthy NJ. Critical review of the management of early-stage breast cancer in elderly women. Intern Med J. 2007;37:181–9.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83.
Schmidinger M, et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2008;26:5204–12.
Lonardi S, Bortolami A, Stefani M, Monfardini S. Oral anticancer drugs in the elderly: an overview. Drugs Aging. 2007;24:395–410.
Bayer HealthCare Pharmaceuticals, Inc. Nexavar: highlights of prescribing information. 2009. Available at: http://www.univgraph.com/bayer/inserts/nexavar.pdf. Accessed 16 Mar 2009.
Gebhardt MW, Governali JF, Hart EJ. Drug-related behavior, knowledge, and misconceptions among a selected group of senior citizens. J Drug Educ. 1978;8:85–92.
Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–97.
Bayer HealthCare Pharmaceuticals, Inc. REACH Program. 2009. Available at: http://www.bayeroncology.com/patient_resources/reach.jsp. Accessed 23 Mar 2009.
Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–86.
Katz S, Akpom CA. A measure of primary sociobiological functions. Int J Health Serv. 1976;6:493–508.
Wedding U, Pientka L, Höffken K. Quality-of-life in elderly patients with cancer: a short review. Eur J Cancer. 2007;43:2203–10.
Porta C, Paglino C, Imarisio I, Bonomi L. Uncovering Pandora’s vase: the growing problem of new toxicities from novel anticancer agents. The case of sorafenib and sunitinib. Clin Exp Med. 2007;7:127–34.
Kollmannsberger C, et al. Sunitinib therapy for metastatic renal cell carcinoma: recommendations for management of side effects. Can Urol Assoc J. 2007;1(2 suppl):S41–54.
Balducci L. Epidemiology of anemia in the elderly: information on diagnostic evaluation. J Am Geriatr Soc. 2003;51(3 suppl):S2–9.
Gastrointestinal disorders: constipation, diarrhea, and fecal incontinence. In: Beers MH, Berkow R, editors. The Merck manual of geriatrics. Whitehouse Station: Merck Research Laboratories; 2000. pp. 1080–1094.
Lacouture ME, et al. Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist. 2008;13:1001–11.
Lacouture ME, et al. Inflammation of actinic keratoses subsequent to therapy with sorafenib, a multitargeted tyrosine-kinase inhibitor. Clin Exp Dermatol. 2006;31:783–5.
Kong HH, et al. Keratoacanthomas associated with sorafenib therapy. J Am Acad Dermatol. 2007;56:171–2.
Autier J, Escudier B, Wechsler J, Spatz A, Robert C. Prospective study of the cutaneous adverse effects of sorafenib, a novel multikinase inhibitor. Arch Dermatol. 2008;144:886–92.
Dubauskas Z, et al. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20–3.
Arnault JP, et al. Keratoacanthomas and squamous cell carcinomas in patients receiving sorafenib. J Clin Oncol. 2009;27(23):e59–61.
Wood LS, Manchen B. Sorafenib: a promising new targeted therapy for renal cell carcinoma. Clin J Oncol Nurs. 2007;11:649–56.
Morley JE. Pathophysiology of anorexia. Clin Geriatr Med. 2002;18:661–73.
Tchekmedyian NS, Zahyna D, Halpert C, Heber D. Clinical aspects of nutrition in advanced cancer. Oncology. 1992;49(suppl 2):3–7.
Golden AG, Daiello LA, Silverman MA, Llorente M, Preston RA. University of Miami Division of Clinical Pharmacology Therapeutic Rounds: medications used to treat anorexia in the frail elderly. Am J Ther. 2003;10:292–8.
McNeil JJ, Silagy CA. Hypertension in the elderly: epidemiology and pathophysiology. Cardiovasc Drugs Ther. 1991;4(suppl 6):1197–201.
World Health Organization, International Society of Hypertension Writing Group. World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21:1983–92.
Acknowledgments
The development of this article was supported by Bayer HealthCare Pharmaceuticals. This article is based upon an expert panel discussion with the authors held on February 26, 2009, supported by Bayer HealthCare. Envision Communications, LLC, provided writing and editorial support in the development of this article, funded by Bayer HealthCare.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Disclosures/Conflicts of interest | JPD | NT | JB | BE |
|---|---|---|---|---|
Employment/leadership position | – | – | – | – |
Intellectual property rights/inventor/patent holder | – | – | – | – |
Consultant/advisory role | b,c,e,g | – | – | a,e,f |
Honoraria | b,d,e,g | b | a,d,e–g | a,c–f |
Research funding/contracted research | b–e,g | – | – | – |
Ownership interest | – | – | – | – |
Expert testimony | – | – | – | – |
Other | – | – | – | – |
Rights and permissions
About this article
Cite this article
Dutcher, J.P., Tannir, N., Bellmunt, J. et al. Experience with sorafenib and the elderly patient. Med Oncol 27, 1359–1370 (2010). https://doi.org/10.1007/s12032-009-9388-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-009-9388-4