Abstract
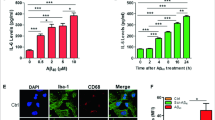
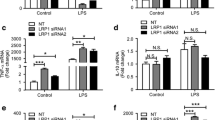
It is well known that extracellular deposition of amyloid-β (Aβ) peptide and microglia-mediated neuroinflammation are major hallmarks of Alzheimer’s disease (AD). Interferon regulatory factor-8 (IRF-8), an important transcription factor of the IRF family, is highly restricted in microglia in brains. The expression pattern and function of IRF-8 in AD need to be elucidated in order to provide novel therapies for the treatment of AD. In this study, our results indicated that expression of IRF-8 is significantly elevated in the brains and microglia of AD transgenic model Tg2576 mice. Notably, in vitro cell culture and reporter luciferase assay show that Aβ1–40 treatment promotes expression of IRF-8 at the transcriptional level. Silencing of IRF-8 in microglia abolished Aβ1–40-induced elevation in typical activated microglia-related genes, including the microglial innate response receptor toll-like receptor 2 (TLR2), the chemotaxis gene purinergic receptor P2Y12R, and the proinflammatory cytokine IL-1β. However, overexpression of IRF-8 exacerbated the elevated expression of these proteins. Finally, the JAK2/STAT-1 pathway was found to mediate Aβ1–40-induced elevation of IRF-8. Overall, this is the first time to report that IRF-8 is involved in microglial activation and neuroinflammation in AD.




Similar content being viewed by others
References
Akiyama H, Mori H, Saido T, Kondo H, Ikeda K, McGeer PL (1999) Occurrence of the diffuse amyloid β-protein (Aβ) deposits with numerous Aβ-containing glial cells in the cerebral cortex of patients with Alzheimer’s disease. Glia 25:324–331
An YW, Jhang YW, Woo SY, Kang SY, Chong SY (2016) Sulforaphane exerts its anti-inflammatory effect against amyloid-β peptide via STAT-1 dephosphorylation and activation of Nrf2/HO-1 cascade in human THP-1 macrophages. Neurobiol Aging 38:1–10
Benzing WC, Wujek WC, Ward EK, Shaffer D, Ashe KH, Younkin SG, Brunden KR (1999) Evidence for glial-mediated inflammation in aged APP(SW) transgenic mice. Neurobiol Aging 20(6):581–589
Cho HJ, Kim SK, Jin SM, Hwang EM, Kim YS, Huh K, Mook-Jung I (2007) IFN-gamma-induced BACE1 expression is mediated by activation of JAK2 and ERK1/2 signaling pathways and direct binding of STAT1 to BACE1 promoter in astrocytes. Glia 55:253–262
Daniilidou M, Koutroumani M, Tsolaki M (2011) Epigenetic mechanisms in Alzheimer’s disease. Curr Med Chem 18:1751–1756
Galimberti D, Scarpini D (2011) Inflammation and oxidative damage in Alzheimer’s disease: friend or foe? Front Biosci (Schol Ed) 3:252–266
Horiuchi M, Wakayama M, Itoh A, Kawai A, Pleasure D, Ozato D, Itoh T (2012) Interferon regulatory factor 8/interferon consensus sequence binding protein is a critical transcription factor for the physiological phenotype of microglia. J Neuroinflammation 9:227
Inoue K (2006) The function of microglia through purinergic receptors: neuropathic pain and cytokine release. Pharmacol Ther 109:210–226
Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y (1994) Visualization of Aβ42(43) and Aβ40 in senile plaques with end-specific Aβ monoclonals: evidence that an initially deposited species is Aβ42(43). Neuron 13:45–53
Jana M, Palencia CA, Pahan K (2008) Fibrillar amyloid-beta peptides activate microglia via TLR2: implications for Alzheimer’s disease. J Immunol 181:7254–7262
Jiang D, Zhang L, Grant GPG, Dudzik CG, Chen S, Patel S, Hao Y, Millhauser GL, Zhou F (2013) The elevated copper binding strength of amyloid-β aggregates allows the sequestration of copper from albumin: a pathway to accumulation of copper in senile plaques. Biochemistry 52:547–556
Kim OS, Park OS, Joe EH, Jou I (2002) JAK-STAT signaling mediates gangliosides-induced inflammatory responses in brain microglial cells. J Biol Chem 277:40594–40601
Lin W, Ding W, Xue J, Leng W, Lin W, Ding M et al (2013) The role of TLR2/JNK/NF-κB pathway in amyloid β peptide-induced inflammatory response in mouse NG108-15 neural cells. Int Immunopharmacol 17:880-4.v
Lue LF, Kuo YM, Roher AE et al (1999) Soluble amyloid β peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am J Pathol 155:853–862
Maccioni RB, Morales I, Guzman-Martinez L, Cerda-Troncoso C, Farías GA (2014) Neuroinflammation in the pathogenesis of Alzheimer’s disease. A rational framework for the search of novel therapeutic approaches. Front. Cell. Neurosci. 8:1–9
Masuda T, Tsuda M, Yoshinaga R, Tozaki-Saitoh H, Ozato H, Tamura T, Inoue K (2012a) IRF8 is a critical transcription factor for transforming microglia into a reactive phenotype. Cell Rep 1:334–340
Masuda T, Tsuda T, Yoshinaga R, Tozaki-Saitoh R, Ozato K, Tamura K et al (2012b) IRF8 is a critical transcription factor for transforming microglia into a reactive phenotype. Cell Rep 1:334–340
Masuda T, Tsuda T, Yoshinaga R, Tozaki-Saitoh R, Ozato K, Tamura K et al (2012c) IRF8 is a critical transcription factor for transforming microglia into a reactive phenotype. Cell Rep 1:334–340
Masuda T, Iwamoto T, Mikuriya S, Tozaki-Saitoh S, Tamura T, Tsuda T, Inoue K (2015) Transcription factor IRF1 is responsible for IRF8-mediated IL-1β expression in reactive microglia. J Pharmacol Sci 128:216–220
McGeer PL, Rogers PL, McGeer EG (2006) Inflammation, anti-inflammatory agents and Alzheimer disease: the last 12 years. J Alzheimers Dis 9:271–276
Strooper BD (2010) Proteases and proteolysis in Alzheimer disease: a multifactorial view on the disease process. Physiol Rev 90:465–494
Takata K, Kitamura Y, Umeki M et al (2003) Possible involvement of small oligomers of amyloid-β peptides in 15-deoxy-Δ12,14 prostaglandin J2-sensitive microGlial activation. J Pharmacol Sci 91:330–333
Funding Information
This study was supported by the Science and Technology Planning Project of Guangdong Province, China (2016ZC0062) and Medical Scientific Research Foundation of Guangdong Province, China (A2015153).
Author information
Authors and Affiliations
Corresponding author
Additional information
Qinggan Zeng, Rongyong Man, and Yifeng Luo are co-first authors.
Rights and permissions
About this article
Cite this article
Zeng, Q., Man, R., Luo, Y. et al. IRF-8 is Involved in Amyloid-β1–40 (Aβ1–40)-induced Microglial Activation: a New Implication in Alzheimer’s Disease. J Mol Neurosci 63, 159–164 (2017). https://doi.org/10.1007/s12031-017-0966-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-017-0966-1