Abstract
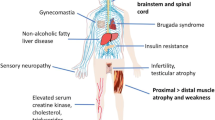
Spinal and bulbar muscular atrophy (SBMA) is an X-linked neuromuscular disease characterized by late-onset, progressive degeneration of lower motor neurons and skeletal muscle atrophy. SBMA is caused by expansions of a CAG trinucleotide repeat in the gene encoding the androgen receptor (AR). One striking feature of SBMA is sex specificity: SBMA fully manifests only in males, whereas females show subclinical or mild disease manifestations even when homozygous for the mutation. Since the identification of the mutation responsible for SBMA in 1991, several cell and animal models have been developed to recapitulate the main features of disease in vitro and in vivo. In this review, we describe the most widely used cellular and animal models of SBMA, highlighting advantages and disadvantages in the use of these models to gain mechanistic and therapeutic insights into SBMA.
Similar content being viewed by others
References
Albertelli MA, Scheller A, Brogley M, Robins DM (2006) Replacing the mouse androgen receptor with human alleles demonstrates glutamine tract length-dependent effects on physiology and tumorigenesis in mice. Mol Endocrinol 20(6):1248–1260
Biedler JL, Helson L, Spengler BA (1973) Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res 33(11):2643–2652
Brooks BP, Paulson HL, Merry DE et al (1997) Characterization of an expanded glutamine repeat androgen receptor in a neuronal cell culture system. Neurobiol Dis 3(4):313–323
Brooks BP, Merry DE, Paulson HL, Lieberman AP, Kolson DL, Fischbeck KH (1998) A cell culture model for androgen effects in motor neurons. J Neurochem 70(3):1054–1060
Chevalier-Larsen ES, O’Brien CJ, Wang H et al (2004) Castration restores function and neurofilament alterations of aged symptomatic males in a transgenic mouse model of spinal and bulbar muscular atrophy. J Neurosci 24(20):4778–4786
Chua JP, Reddy SL, Merry DE et al (2014) Transcriptional activation of TFEB/ZKSCAN3 target genes underlies enhanced autophagy in spinobulbar muscular atrophy. Hum Mol Genet 23(5):1376–1386
Cortes CJ, Ling SC, Guo LT et al (2014a) Muscle expression of mutant androgen receptor accounts for systemic and motor neuron disease phenotypes in spinal and bulbar muscular atrophy. Neuron 82(2):295–307
Cortes CJ, Miranda HC, Frankowski H et al (2014b) Polyglutamine-expanded androgen receptor interferes with TFEB to elicit autophagy defects in SBMA. Nat Neurosci 17(9):1180–1189
Dossena M, Bedini G, Rusmini P et al (2014) Human adipose-derived mesenchymal stem cells as a new model of spinal and bulbar muscular atrophy. PLoS One 9(11), e112746
Durham HD, Dahrouge S, Cashman NR (1993) Evaluation of the spinal cord neuron X neuroblastoma hybrid cell line NSC-34 as a model for neurotoxicity testing. Neurotoxicology 14(4):387–395
Greene LA, Tischler AS (1976) Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A 73(7):2424–2428
Grunseich C, Kats IR, Bott LC et al (2014a) Early onset and novel features in a spinal and bulbar muscular atrophy patient with a 68 CAG repeat. Neuromuscul Disord 24(11):978–981
Grunseich C, Zukosky K, Kats IR et al (2014b) Stem cell-derived motor neurons from spinal and bulbar muscular atrophy patients. Neurobiol Dis 70C:12–20
Jochum T, Ritz ME, Schuster C et al (2012) Toxic and non-toxic aggregates from the SBMA and normal forms of androgen receptor have distinct oligomeric structures. Biochim Biophys Acta 1822(6):1070–1078
Katsuno M, Adachi H, Kume A et al (2002) Testosterone reduction prevents phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Neuron 35(5):843–854
Katsuno M, Adachi H, Doyu M et al (2003) Leuprorelin rescues polyglutamine-dependent phenotypes in a transgenic mouse model of spinal and bulbar muscular atrophy. Nat Med 9(6):768–773
La Spada AR, Wilson EM, Lubahn DB, Harding AE, Fischbeck KH (1991) Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature 352(6330):77–79
Landis SC, Amara SG, Asadullah K et al (2012) A call for transparent reporting to optimize the predictive value of preclinical research. Nature 490(7419):187–191
Malena A, Pennuto M, Tezze C et al (2013) Androgen-dependent impairment of myogenesis in spinal and bulbar muscular atrophy. Acta Neuropathol 126(1):109–121
Monks DA, Johansen JA, Mo K et al (2007) Overexpression of wild-type androgen receptor in muscle recapitulates polyglutamine disease. Proc Natl Acad Sci U S A 104(46):18259–18264
Nedelsky NB, Pennuto M, Smith RB et al (2010) Native functions of the androgen receptor are essential to pathogenesis in a Drosophila model of spinobulbar muscular atrophy. Neuron 67(6):936–952
Olmsted JB, Carlson K, Klebe R, Ruddle F, Rosenbaum J (1970) Isolation of microtubule protein from cultured mouse neuroblastoma cells. Proc Natl Acad Sci U S A 65(1):129–136
Palazzolo I, Burnett BG, Young JE et al (2007) Akt blocks ligand binding and protects against expanded polyglutamine androgen receptor toxicity. Hum Mol Genet 16(13):1593–1603
Palazzolo I, Stack C, Kong L et al (2009) Overexpression of IGF-1 in muscle attenuates disease in a mouse model of spinal and bulbar muscular atrophy. Neuron 63(3):316–328
Palazzolo I, Nedelsky NB, Askew CE et al (2010) B2 attenuates polyglutamine-expanded androgen receptor toxicity in cell and fly models of spinal and bulbar muscular atrophy. J Neurosci Res 88(10):2207–2216
Pandey UB, Nie Z, Batlevi Y et al (2007) HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature 447(7146):859–863
Parodi S, Pennuto M (2011) Neurotoxic effects of androgens in spinal and bulbar muscular atrophy. Front Neuroendocrinol 32(4):416–425
Ramzan F, McPhail M, Rao P et al (2015) Distinct etiological roles for myocytes and motor neurons in a mouse model of Kennedy’s disease/spinobulbar muscular atrophy. J Neurosci 35(16):6444–6451
Ranganathan S, Harmison GG, Meyertholen K, Pennuto M, Burnett BG, Fischbeck KH (2009) Mitochondrial abnormalities in spinal and bulbar muscular atrophy. Hum Mol Genet 18(1):27–42
Rao P, Monks DA (2009) A tetracycline-inducible and skeletal muscle-specific Cre recombinase transgenic mouse. Dev Neurobiol 69(6):401–406
Salazar-Grueso EF, Kim S, Kim H (1991) Embryonic mouse spinal cord motor neuron hybrid cells. Neuroreport 2(9):505–508
Sambataro F, Pennuto M (2012) Cell-autonomous and non-cell-autonomous toxicity in polyglutamine diseases. Prog Neurobiol 97(2):152–172
Scaramuzzino C, Casci I, Parodi S et al (2015) Protein arginine methyltransferase 6 enhances polyglutamine-expanded androgen receptor function and toxicity in spinal and bulbar muscular atrophy. Neuron 85(1):88–100
Sheppard RL, Spangenburg EE, Chin ER, Roth SM (2011) Androgen receptor polyglutamine repeat length affects receptor activity and C2C12 cell development. Physiol Genomics 43(20):1135–1143
Simeoni S, Mancini MA, Stenoien DL et al (2000) Motoneuronal cell death is not correlated with aggregate formation of androgen receptors containing an elongated polyglutamine tract. Hum Mol Genet 9(1):133–144
Sopher BL, Thomas PS Jr, LaFevre-Bernt MA et al (2004) Androgen receptor YAC transgenic mice recapitulate SBMA motor neuronopathy and implicate VEGF164 in the motor neuron degeneration. Neuron 41(5):687–699
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–676
Takeyama K, Ito S, Yamamoto A et al (2002) Androgen-dependent neurodegeneration by polyglutamine-expanded human androgen receptor in Drosophila. Neuron 35(5):855–864
Walcott JL, Merry DE (2002) Ligand promotes intranuclear inclusions in a novel cell model of spinal and bulbar muscular atrophy. J Biol Chem 277(52):50855–50859
Xu Z, Joel Tito A, Rui YN, Zhang S (2015) Studying polyglutamine diseases in Drosophila. Exp Neurol
Yaffe D, Saxel O (1977) Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 270(5639):725–727
Yu Z, Dadgar N, Albertelli M et al (2006) Androgen-dependent pathology demonstrates myopathic contribution to the Kennedy disease phenotype in a mouse knock-in model. J Clin Invest 116(10):2663–2672
Acknowledgments
This work was supported by the Telethon-Italy and Provincia Autonoma di Trento (TCP12013 to M.P.), the Italian Ministry of Health (RF-2011-02350097 to M.P.), the French Muscular Dystrophy Association (18722 to M.P.), the Associazione Alzheimer Trento Onlus (to M.B.), the Bando Progetti Strategici di Ateneo-University of Trento (to M.P. and M.B.), and CIBIO internal funds.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical Approval
Animal care and experimental procedures were conducted in accordance with the University of Trento ethical committee and were approved by the Italian Ministry of Health.
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Pennuto, M., Basso, M. In Vitro and In Vivo Modeling of Spinal and Bulbar Muscular Atrophy. J Mol Neurosci 58, 365–373 (2016). https://doi.org/10.1007/s12031-015-0677-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-015-0677-4