Abstract
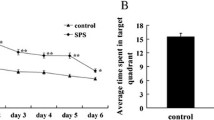
Posttraumatic stress disorder (PTSD) is an anxiety disorder caused by a life-threatening traumatic experience, which affects a patient’s quality of life and social stability. The objective of this study was to investigate the change of the glucose-regulated protein (GRP) 94 and apoptosis-related caspase-12 expression in medial prefrontal cortex (mPFC) in rats to determine whether endoplasmic reticulum apoptosis pathway plays an important role in single-prolonged stress (SPS), a well-established PTSD animal model, and therefore to provide experimental evidence to reveal PTSD pathogenesis. A total of 120 healthy male Wistar rats were selected for this study, randomly divided into a normal control group and SPS groups of 1, 4, 7, 14, and 28 days. Behavioral studies of the learning and memory capabilities of rats were observed by using Morris water maze. Morphological changes were detected using transmission electron microscopy (TEM). Immunohistochemistry, Western blot, and reverse transcription polymerase chain reaction (RT-PCR) were used to detect the expressions of caspase-12 and GRP94 expressions in mPFC. Our results showed that compared with control groups, after the SPS exposure, the average escape latency was prolonged in place navigation test (P < 0.05), and swimming time in the third quadrant in spatial probe test shortened. The morphological change of mPFC in each SPS group bears typical apoptotic characteristics. The expressions of GRP94 and caspase-12 gradually increased on 1 and 4 days, peaked on 7 days after the SPS exposure, and then decreased. These results suggest that SPS exposure can induce apoptotic neurons and a change of caspase-12 and GRP94 expression in the mPFC, which may be one of the pathogenesis of mPFC abnormal function in PTSD.






Similar content being viewed by others
References
American psychiatric association: diagnostic and statistical manual of mental disorders (1994) DSM-IV, 4th edn. American Psychiatric Press, Washington
Bernales S, Papa FR, Walter P (2006) Intracellular signaling by the unfolded protein response. Annu Rev Cell Dev Biol 22:487–508
Chang YJ, Tai CJ, Kuo LJ et al (2011) Glucose–regulated protein 78 (GRP78) mediated the efficacy to curcumin treatment on hepatocellular carcinoma. Ann Surg Oncol 18:2395–2403
Damasio H, Grabowski T, Frank R et al (1994) The return of Phineas Gage: clues about the brain from the skull of a famous patient. Science 264:1102–1105
Eckart C, Stoppel C, Kaufmann J et al (2011) Structural alterations in lateral prefrontal, parietal and posterior midline regions of men with chronic posttraumatic stress disorder. J Psychiatry Neurosci 36:176–186
Frick KM, Baxter MG, Markowaska AL et al (1995) Age-related spatial reference and working memory deficits assessed in the water maze. Neurobiol Aging 16:149–160
Furay AR, Bruestle AE, Herman JP (2008) The tole of the forebrain glucocorticoid recepror in acute and chronic srees. Endocrinology 149(11):5482–5490
Harvey AG, Bryant RA (2002) Acute stress disorder: a synthesis and critique. Psychol Bull 128(6):886–902
Hoge CW, Castro CA, Messer SC et al (2004) Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med 351:13–22
Hull AM (2002) Neuroimaging findings in post-traumatic stress disorder. Systematic review. Br J Psychiatry 181:102–110
Kessler RC (2000) Post-traumatic stress disorder the burden to the individual and to society. J Clin psychiatrt 61(suppl5) 4–12
Kohda K, Harada K, Kato K et al (2007) Glucocorticoid receptor activation is involved in producing abnormal phenotypes of single–prolonged stress rats: a putative post-traumatic stress disorder model. Neuroscience 148:22–33
Lin XY, Choi MS, Porter AG (2000) Expression analysis of the human caspase-1 subfamily reveals specific regulation of the CASP5 gene by lipopolys accharide and interferon–gamma. J Biol Chem 275:39920–39926
Li Y, Han F, Shi Y (2013) Increased neuronal apoptosis in medial prefrontal cortex is accompanied with changes of Bcl-2 and Bax in a rat model of post-traumatic stress disorder. J Mol Neurosci 32:625–631
Morris R (1981) Spatial localisation does not depend on the presence of local cues. Learn Motiv 12:239–260
Morris RGM (2008) Morris water maze. Scholarpedia 3:6315
Nakagawa T, Zhu H, Morishima N et al (2000) Caspase-12 mediates endoplasmic–reticulum specific apoptosis and cytotoxicity by amyloid-beta. Nature 403:98–103
Nieves D, Moreno JJ (2007) Epoxyeicosatrienoic acids induce growth inhibition and calpain/caspase-12 dependent apoptosis in PDGF cultured 3T6 fibroblast. Apoptosis 12(11):1979–1988
Patil C, Walter P (2001) Intracellular signaling from the endoplasmic reticulum to the nucleus: the unfolded protein response in yeast, and mammals. Curr Opin Cell Biol 13:349–355
Pollice R, Bianchini V, Roncone R et al (2012) Psychological distress and post-traumatic stress disorder (PTSD) in young survivors of L'Aquila earthquake. Rev Psichiatr 47:59–64
Roozendaal B, McReynolds JR, Van der Zee EA et al (2009) Glucocorticoid effects on memory consolidation depend on functional interactions between the medial prefrontal cortex and basolateral amygdala. J Neurosci 29:14299–308
Shalev AY (2002) Acute stress reactions in adults. Biol Psychiatry 51(7):532–543
Sherin JE, Nemeroff CB (2011) Post-traumatic stress disorder: the neurobiological impact of psychological trauma. Dialogues Clin Neurosci 13:263–278
Shin LM, Liberzon I (2010) The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 35:169–191
Shin LM, Rauch SL, Pitman RK et al (2006) Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci 1071:67–79
Sreedhar AS, Kalmar E, Csermely P et al (2004) Hsp90 isoforms: functions, expression and clinical importance. FEBS Lett 562:11–15
Shibata M, Hattori H, Sasaki T (2003) Activation of caspase-12 by endoplasmic reticulum stress induced by transient middle cerebral artery occlusion in mice. Neuroscience 118:491–499
Takahashi T, Morinobu S, Iwamoto Y et al (2006) Effect of paroxetine on enhanced contextual fear induced by single prolonged stress in rats. Psychopharmacol (Ber) 189:165–173
Wang HT, Han F, Gao JL, Shi YX (2010) Increased phosphorylation of extracellular signal–regulated kinase in the medial prefrontal cortex of the single-prolonged stress rats. Cell Mol Neurobiol 30:437–444
Wen Y, LI B, Han F et al (2012) Dysfunction of calcium/calmodulin/CaM kinase IIα cascades in the medial prefrontal cortex in post-traumatic stress disorder. Mol Med Rep 6:1140–1144
Whitesell L, Bagatell R, Falsey R (2003) The stress response: implications for the clinical development of hsp90 inhibitors. Cancer Drug Targets 3:349–358
Xiao B, Yu B, Wang H–T et al (2011) Single-prolonged stress induces apoptosis by activating cytochrome C/caspase-9 pathway in a rat model of posttraumatic stress disorder. Cell Mol Neurobiol 31:37–43
Yehuda R (2005) Neuroendocrine aspects of PTSD. Handb Exp Pharmacol 169:371–403
Zhu CZ, Situ MJ, Zhang Y et al (2011) Influence factors of posttraumatic stress disorder (PTSD) and depression symptoms in children and adolescents after Wenchuan earthquake in China. Zhonghua Yu Fang Yi Xue Za Zhi 45:531–536
Acknowledgments
This research was supported by grants from the National Natural Science Foundation of China (No. 81171282; No. 31200772) and research fund from the doctoral program of High Education of China (No. 20132104110021). The authors would like to thank the reviewers for their valuable comments on how to improve the quality of the paper.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, D., Han, F. & Shi, Y. Effect of Glucose-Regulated Protein 94 and Endoplasmic Reticulum Modulator Caspase-12 in Medial Prefrontal Cortex in a Rat Model of Posttraumatic Stress Disorder. J Mol Neurosci 54, 147–155 (2014). https://doi.org/10.1007/s12031-014-0263-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-014-0263-1