Abstract
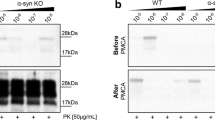
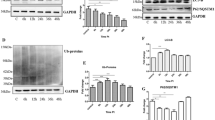
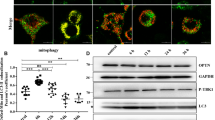
Prion diseases characterize a category of fatal neurodegenerative diseases. Although reports have increasingly shown that oxidative stress plays an important role in the progression of prion diseases, little is known about whether oxidative stress is a cause or a consequence of a prion disease. The mechanism of prion disease development also remains unclear. The purpose of this study was to investigate three things: the possible mechanisms of neuron cell damage, the conformation of anti-protease K (PK) PrPSc, and the role of oxidative stress in the progression of prion diseases. The study results demonstrated that normal PrPC transformed into a PK-resistant protein under oxidative stress in the presence of PrP106–126. Further, the protein misfolding cyclic amplification procedure may have accelerated this process. Mitochondrial damage and dysfunction in prion disease progression were also observed in this study. Our results suggested that neuron cell damage, and particularly mitochondrial damage, was induced by oxidative stress. This damage may be the initial cause of a given prion disease.




Similar content being viewed by others
References
Bocharova OV, Breydo L, Parfenov AS, Salnikov VV, Baskakov IV (2005) In vitro conversion of full-length mammalian prion protein produces amyloid form with physical properties of PrP Sc. J Mol Biol 346(2):645–659
Brazier MW, Lewis V, Ciccotosto GD, Klug GM, Lawson VA, Cappai R, Ironside JW, Masters CL, Hill AF, White AR (2006) Correlative studies support lipid peroxidation is linked to PrPres propagation as an early primary pathogenic event in prion disease. Brain Res Bull 68(5):346–354
Castilla J, Saá P, Morales R, Abid K, Maundrell K, Soto C (2006) Protein misfolding cyclic amplification for diagnosis and prion propagation studies. Method Enzymol 412:3–21
Chen B, Morales R, Barria MA, Soto C (2010) Estimating prion concentration in fluids and tissues by quantitative PMCA. Nat methods 7(7):519–520
Choi CJ, Kanthasamy A, Anantharam V, Kanthasamy AG (2006) Interaction of metals with prion protein: possible role of divalent cations in the pathogenesis of prion diseases. NeuroToxicology 27(5):777–787
Dlasková A, Hlavatá L, Ježek P (2008) Oxidative stress caused by blocking of mitochondrial Complex I H+ pumping as a link in aging/disease vicious cycle. Int J Biochem Cell Biol 40(9):1792–1805
Fioriti L, Quaglio E, Massignan T, Colombo L, Stewart RS, Salmona M, Harris DA, Forloni G, Chiesa R (2005) The neurotoxicity of prion protein (PrP) peptide 106–126 is independent of the expression level of PrP and is not mediated by abnormal PrP species. Mol Cell Neurosci 28(1):165–176
Haigh CL, McGlade AR, Lewis V, Masters CL, Lawson VA, Collins SJ (2011) Acute exposure to prion infection induces transient oxidative stress progressing to be cumulatively deleterious with chronic propagation in vitro. Free Radical Bio Med 51:594–608
Kim NH, Park SJ, Jin JK, Kwon MS, Choi EK, Carp RI, Kim YS (2000) Increased ferric iron content and iron-induced oxidative stress in the brains of scrapie-infected mice. Brain Res 884(1–2):98–103
Kozlowski H, Janicka-Klos A, Brasun J, Gaggelli E, Valensin D, Valensin G (2009) Copper, iron, and zinc ions homeostasis and their role in neurodegenerative disorders (metal uptake, transport, distribution and regulation). Coordin Chem Rev 253(21):2665–2685
Ma J, Lindquist S (1999) De novo generation of a PrPSc-like conformation in living cells. Nat Cell Biol 1(6):358–361
Milhavet O, Lehmann S (2002) Oxidative stress and the prion protein in transmissible spongiform encephalopathies. Brain Res Rev 38(3):328–339
Panza G, Stöhr J, Dumpitak C, Papathanassiou D, Weiß J, Riesner D, Willbold D, Birkmann E (2008) Spontaneous and BSE-prion-seeded amyloid formation of full length recombinant bovine prion protein. Biochem Bioph Res Co 373(4):493–497
Prusiner SB (1982) Novel proteinaceous infectious particles cause scrapie. Science 216(4542):136–144
Saá P, Castilla J, Soto C (2005) Cyclic amplification of protein misfolding and aggregation. Methods Mol Biol 299:53–65
Saretzki G (2009) Telomerase, mitochondria and oxidative stress. Exp Gerontol 44(8):485–492
Seo JS, Seol JW, Moon MH, Jeong JK, Lee YJ, Park SY (2010) Hypoxia protects neuronal cells from human prion protein fragment–induced apoptosis. J Neurochem 112(3):715–722
Sun K, Johnson BS, Gunn TM (2007) Mitochondrial dysfunction precedes neurodegeneration in mahogunin (Mgrn1) mutant mice. Neurobiol Aging 28(12):1840–1852
Valente EM, Abou-Sleiman PM, Caputo V, Muqit MMK, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG (2004) Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science 304(5674):1158–1160
Zheng W, Wang L, Hong Y, Sha Y (2009) PrP106-126 peptide disrupts lipid membranes: influence of C-terminal amidation. Biochem Bioph Res Co 379(2):298–303
Acknowledgments
This work was supported by the Natural Science Foundation of China (project no. 31001048 and no. 31172293), Specialized Research Fund for the Doctoral Program of Higher Education (SRFDP, project no. 20100008120002), and the Foundation of Chinese Ministry of Science and Technology (project no. 2011BAI15B01), and the Program for Cheung Kong Scholars and Innovative Research Team in University of China (no. IRT0866).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yuan, F., Yang, L., Zhang, Z. et al. Cellular Prion Protein (PrPC) of the Neuron Cell Transformed to a PK-Resistant Protein Under Oxidative Stress, Comprising Main Mitochondrial Damage in Prion Diseases. J Mol Neurosci 51, 219–224 (2013). https://doi.org/10.1007/s12031-013-0008-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-013-0008-6