Abstract
The SLC30 family of divalent cation transporters is thought to be involved in the transport of zinc in a variety of cellular pathways. Zinc transporter 3 (ZnT3) is involved in the transport of zinc into synaptic vesicles or intracellular organelles. As the presence of ZnT3 immunoreactive neurons has recently been reported in both the central and peripheral nervous systems of the rat, the present study was aimed at disclosing the presence of a zinc-enriched neuron enteric population in the porcine duodenum to establish a preliminary insight into their neurochemical coding. Double- and triple-immunofluorescence labeling of the porcine duodenum for ZnT3 with the pan-neuronal marker (PGP 9.5), substance P, somatostatin, vasoactive intestinal peptide (VIP), nitric oxide synthase (NOS), leu-enkephalin, vesicular acetylcholine transporter (VAChT), neuropeptide Y, galanin (GAL), and calcitonin gene-related peptide were performed. Immunohistochemistry revealed that approximately 35, 43, and 48 % of all PGP9.5-postive neurons in the myenteric (MP), outer submucous (OSP), and inner submucous (ISP) plexuses, respectively, of the porcine duodenum were simultaneously ZnT3+. In the present study, ZnT3+ neurons coexpressed a broad spectrum of active substances, but co-localization patterns unique to the plexus were studied. In the ISP, all ZnT3+ neurons were VAChT positive, and the largest populations among these cells formed ZnT3+/VAChT+/GAL+ and ZnT3+/VAChT+/VIP+ cells. In the OSP and MP, the numbers of ZnT3+/VAChT+ neurons were two times smaller, and substantial subpopulations of ZnT3+ neurons in both these plexuses formed ZnT3+/NOS+ cells. The large population of ZnT3+ neurons in the porcine duodenum and a broad spectrum of active substances which co-localize with this peptide suggest that ZnT3 takes part in the regulation of various processes in the gut both in normal physiology and during pathological processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well known that local intestinal activity, such as muscle contraction and relaxation or mucosal ion secretion, is controlled by the enteric nervous system (ENS). The different functions of the ENS are controlled by more than 20 classes of neurons, each with a diverse variety of co-localized and co-released neurotransmitters that are unique to their target cell receptors (Brown and Timmermans 2004; Furness 2000). The porcine ENS displays considerable similarities to that of humans in respect of anatomical, physiological, and pathological characteristics, and it is organized into three layers called plexuses: myenteric plexus (MP), outer submucous plexus (OSP), and inner submucous plexus (ISP). The MP is located between the longitudinal and circular muscle layers, and the majority of these myenteric neurons takes part in the control of digestive motility (Huizinga et al. 2011), but a small subpopulation of these neurons supplies the submucosal plexuses and/or regulates the secretory functions of the gut (Brehmer et al. 1994). Neurons of the OSP (located near the internal part of the circular muscle layer) and ISP (located between the muscularis mucosa and lamina propria) generally supply the mucosal and submucosal layers and regulate the secretion and intrinsic sensory pathways of the gut in response to luminal contents as well as innervate submucosal blood vessels (Brehmer et al. 2010). However, some neurons, especially in the OSP, can also supply the circular muscle layer of the gut (Scheuermann and Timmermans 1993).
As enteric neurons usually coexpress several active substances that regulate or modulate various cellular processors of the target cell, the chemical coding of the innervating neurons is associated with the functions of the target organ (Brookes 2001; Costa et al. 1996; Furness 2000). Zinc transporter 3 (ZnT3) has been observed in the ENS of the human and porcine species (Gonkowski et al. 2007, 2009). ZnT3 is a member of the SLC 30 family of zinc transporters, which transports zinc ions from the cytoplasm into synaptic vesicles, intracellular organelles, or to the outside of the cell (Palmiter and Huang 2004). However, the role of the Zn transporter in the function of ENS is currently unknown.
The human-ZnT3 protein has 388 amino acids and is predicted to contain six transmembrane domains that form a pore lined with a histidine-rich loop (Gaither and Eide 2001). ZnT3 has been studied in zinc-enriched nerve (ZEN) terminals within the hippocampus, amygdala, neocortex, spinal cord, and superior cervical ganglion neurons (Jo et al. 2000; Wang et al. 2003; Wenzel et al. 1997). The presence of ZnT3 in different regions of the central and peripheral nervous system (Danscher et al. 2003; Wang et al. 2003; Wenzel et al. 1997) suggests that this protein, which is responsible for transporting zinc ions into synaptic vesicles, can thus be used as a marker for tracing ZEN neuronal pathways. Moreover, previous investigations suggest that ZnT3 can play an important role during pathological processes, both within the central nervous system (Frederickson et al. 2000, 2005) and in the ENS (Gonkowski et al. 2007).
Until now, only two studies report the presence of ZnT3 in the ENS of the human (Gonkowski et al. 2009) and the porcine species (Gonkowski et al. 2007). Therefore, the aim of the present study was to investigate for the first time the distribution pattern of ZnT3-immunoreactive (ZnT3+) neurons within the ENS and to quantitatively evaluate the number of ZnT3+ neurons and their chemical coding in the intramural ganglia of the porcine duodenum. Thus, the present study constitutes the introduction to further investigations that should focus on establishing the role of ZnT3 and ZEN nerve functions within the gastrointestinal tract.
Materials and Methods
Study Subjects
Six juvenile and clinically healthy female pigs of the Large White Polish breed (approximately 8 weeks old and 12–15 kg of body weight) were used in the present investigation. The animals were obtained from a commercial fattening farm in Poland. All animals were housed and treated in accordance with rules approved by the ethics committee (conforming to Principles of Laboratory Animal Care, NIH publication no. 86–23, revised 1985). All experimental procedures were approved by the Local Ethics Commission of the University of Warmia and Mazury in Olsztyn (no. 27/2009).
Anesthesia and Surgery
All animals were sedated 30 min prior to the main anesthetic with atropine sulfate (Polfa, Poland; 0.04 mg/kg body weight, s.c.) and azaperone (Stressnil, Janssen Pharmaceutica, Belgium; 2.0 mg/kg body weight, i.m.). All animals were anesthetized with thiobarbital and euthanized by an overdose of the same agent (Thiopental, Sandoz, Austria; 20 mg/kg body weight, i.v.). All animals were then perfused transcardially with 4 % buffered paraformaldehyde (pH 7.4). Following fixative perfusion, small tissue blocks comprising of the duodenum (1 cm from the pylorus) were collected from all studied animals and postfixed by immersion in the same fixative for 4 h, washed twice in 0.1 M phosphate buffer (pH = 7.4, 4 °C) for 3 days, and then stored in 18 % sucrose at 4 °C until sectioning.
Immunofluorescence Experiments
Ten-micrometer-thick cryostat sections of the duodenum samples were processed for routine double- and triple-immunofluorescence labeling using primary antisera raised in different species and species-specific secondary antibodies (Table 1). Frozen sections were removed from the freezer and dried at room temperature for 20 min, rinsed three times in phosphate-buffered saline (PBS), and incubated in a humidified chamber with a blocking buffer (0.1 M PBS, 10 % normal horse serum, 0.01 % bovine serum albumin, 1 % Tween, 0.05 % thimerosal, 0.01 % NaN3) for 1 h. The sections were then rinsed again in PBS and incubated overnight at room temperature with the mixture of primary antibodies using the appropriate combination of antisera: ZnT3 and pan-neuronal marker (PGP 9.5), substance P (SP), somatostatin (SOM), vasoactive intestinal peptide (VIP), nitric oxide synthase (NOS), leu-enkephalin (LENK), vesicular acetylcholine transporter (VAChT), neuropeptide Y (NPY), galanin (GAL), and calcitonin gene-related peptide (CGRP) (Table 1). After the incubation with the primary antibodies, the sections were rinsed in PBS and incubated for 1 h with biotinylated secondary antibodies (during double-labeling immunofluorescence) or with biotinylated 7-amino-4-methylcoumarin-3-acetic acid (AMCA) (during triple-labeling immunofluorescence) (Table 1). After 1 h, the sections were incubated with the mixture of fluorescein isothiocyanate (FITC) and CY3-conjugated streptavidin (Table 1), rinsed again in PBS, then mounted with carbonate-buffered glycerol (pH 8.6) and coverslipped.
Controls
Standard controls, i.e., preabsorption for the neuropeptide antisera (20 mg of appropriate antigen per 1 ml of corresponding antibody at working dilution) and the omission and replacement of all primary antisera by non-immune sera or PBS, were applied to test both antibody and method specificity. To show that ZnT3 found in the enteric system is functional as a Zn transporter, the presence of free or loosely bound zinc ions in enteric neurons was tested using 6-methoxy 8-para toluene sulfonamide quinoline (TSQ) fluorescence and zinc autometallography (AMG) (Fig. 1a-d). Both the TSQ and AMG methods were conducted to show the presence of Zn in the control samples, and they were performed according to instructions described by Wang et al. (2003), Frederickson et al. (1987), and Danscher (1982).
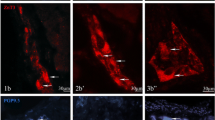
Control staining demonstrating duodenal neurons in the MP (a, a′), OSP (b), and ISP (c) stained with zinc selenide AMG and TSQ methods (d). Some neurons show intensity of AMG staining in the cytoplasm; High-magnification image of two ZnT3+ neurons (e); neurons in the MP, OSP, and ISP of the porcine duodenum immunostained for PGP 9.5 (f, g, h) and ZnT3 (f′, g′, h′). Scale bars = 100 μm (a), 15 μm (a′,b, c, d), 10 μm (e), and 25 μm (f–h′)
Counts and Statistics
The sections were viewed under an Olympus BX51 fluorescence microscope equipped with epifluorescence and appropriate set of filters for FITC, CY3, and AMCA. Microphotographs were acquired with a CCD camera connected by a PC equipped with Olympus image analysis software (ver. 3.2; Soft Imaging System GmbH, Münster, Germany). To determine the percentages of ZnT3+ neurons in each plexus studied (MP, OSP, and ISP), the pan-neuronal marker PGP-9.5 was adopted. PGP-9.5 marks all neurons in the tissue so PGP+/ZnT3+ cells illustrate the percentages of MP, OSP, and ISP neurons coexpressing ZnT3. At least 1,000 PGP-9.5-labeled cell bodies located in 60–70 ganglia of a particular plexus (MP, OSP, and ISP) per animal were examined for ZnT3 immunoreactivity. Only neurons with clearly visible nuclei were counted. To prevent double counting of ZnT3+ neurons, serial sections 200 μm apart were used. Moreover, to determine the percentages of co-localization of ZnT3 with other substances studied, at least 700 ZnT3-positive cell bodies of the same enteric plexus were examined for immunoreactivity to the particular substance investigated. In these double- and triple-labeling studies, ZnT3-positive neurons were considered as 100 % for all combinations, so all the values shown in the text and Table 2 are percentages of ZnT3+ neurons. These data were pooled from all six animals, expressed as means ± standard error of mean (SEM), and then analyzed by using GraphPad Prism 5 software (GraphPad Software, La Jolla, CA, USA).
Results
The Distribution and Number of ZnT3+ Neurons in the Porcine Duodenum
During the present investigation, ZnT3+ cell bodies were observed in all types of enteric plexuses within the porcine duodenum, i.e., in the MP, located between the longitudinal and circular muscle layers; OSP, found in the submucosa; and ISP, located between the muscularis mucosa and lamina propria. The numbers of ZnT3+ neurons were dependent on the plexus studied. The most numerous were found within the ISP (48.2 ± 13.9 %) (Table 2 and Fig. 1h–h′). In the OSP and MP, these neurons constituted 43.0 ± 9.0 and 35.3 ± 8.1 %, respectively (Table 2, Fig. 1f–f′and g–g′). The number of ZnT3+ cells in the single ganglion was 2–5, 2–3, and 5–12 within the MP, OSP, and ISP, respectively. ZnT3+ nerve fibers were not observed in the porcine duodenum.
The Co-localization Pattern of ZnT3+ Neurons in the Porcine Duodenum
In all kinds of duodenal plexuses, the population of ZnT3+ neurons could be subdivided into non-cholinergic (ZnT3+/VAChT−) and cholinergic (ZnT3+/VAChT+) neurons. Both non-cholinergic and cholinergic ZnT3+ neurons coexpressed a broad spectrum of active substances tested in the present study, but co-localization patterns varied depending on the plexus studied (Table 2).
Myenteric Plexus
In the MP, the non-cholinergic and cholinergic ZnT3+ neurons constituted 60.7 ± 1.8 and 40.7 ± 2.9 %, respectively (Table 2). Among the non-cholinergic ZnT3+ neurons, 29.7 ± 3.5 % were also immunoreactive to NOS (Table 2 and Fig. 2a–d). These ZnT3+/NOS+ cells were irregularly dispersed, or they were observed in groups composed of between two and five cells. In addition, 4.3 ± 0.4, 4.4 ± 1.2, 2.7 ± 1.5, and 0.4 ± 0.2 % of the non-cholinergic ZnT3+ neurons were also immunoreactive to SOM (Table 2 and Fig. 2i–l), VIP (Table 2 and Fig. 2e–h), SP (Table 2), and/or LENK (Table 2 and Fig. 2i–l), respectively. Only single ZnT3+/GAL+ and ZnT3+/NPY+ neurons were observed in the porcine duodenal MP while GAL and CGRP coexpression was not observed in the non-cholinergic ZnT3+ myenteric neurons (Table 2). Among the cholinergic ZnT3+ neurons, 2.8 ± 0.8 % were also immunoreactive SOM (Table 2), 2.0 ± 0.9 % were VIP positive (Table 2), and 1.3 ± 0.6 % were SP positive (Table 2). No immunoreactivity to GAL or NOS was observed in these cells (Table 2). It is worth mentioning that ZnT3+ myenteric neurons were supplied with the thick network of VAChT+, LENK+, and/or SP+ nerve fibers and the moderate number of SOM+ and/or VIP+ nerve terminals (Fig. 2). Only a small number of NPY+ and NOS+ nerve fibers and very few CGRP+ nerve terminals were observed around ZnT3+ myenteric neurons.
Representative images of ZnT3+ neurons located in the duodenal MP of the pig. Merged images (d, h, l) are composites of images taken separately from red (a, c, i), green (b, f, j), and blue (c, g, k) fluorescent channels. Scale bar = 25 μm. a–d ZnT3+/VAChT−/NOS+—long arrows, ZnT3+/VAChT+/NOS−—small arrow; e–h ZnT3+/VAChT−/VIP+—long arrow, ZnT3+/VACHT+/VIP−—small arrow; i–l ZnT3+/SOM−/LENK+—long arrow, ZnT3+/SOM+/LENK−—small arrow
Outer Submucosal Plexus
The non-cholinergic ZnT3+ neurons in the OSP constituted 43.1 ± 5.4 (Table 2). Among these neurons, the most numerous were ZnT3+ cells immunoreactive to NOS (19.1 ± 4.8 %; Table 2 and Fig. 3a, c–d). In addition, 10.5 ± 3.1, 4.7 ± 1.6, 3.9 ± 2.5, and 0.4 ± 0.2 % of the non-cholinergic ZnT3+ neurons were also immunoreactive to SOM, VIP, SP, and/or LENK, respectively (Table 2, Fig. 3e–h). On the other hand, none of the non-cholinergic ZnT3+ neurons was ever immunoreactive to GAL, NPY, or CGRP. The cholinergic ZnT3+ neurons in the OSP constituted 56.9 ± 6.4 % (Table 2 and Fig. 3b). The triple-labeling immunofluorescence revealed that 7.4 ± 4.1 % of the cholinergic ZnT3+ neurons in the OSP were also immunoreactive to SOM (Table 2). Moreover, approximately 3.7 ± 1.4 and 4.0 ± 1.7 % were also immunoreactive to VIP and SP (Table 2 and Fig. 3e–h). On the other hand, none of the cholinergic ZnT3+ neurons was ever immunoreactive to GAL and NOS (Table 2 and Fig. 3d). Both the non-cholinergic and cholinergic ZnT3+ neurons were supplied with a thick network of VAChT+ or SOM+ and a moderate number of SP+ or VIP+ nerve fibers (Fig. 3d, h). Only single NOS+ and LENK+ nerve fibers were observed around ZnT3+ neurons within the OSP.
Representative images of ZnT3+ neurons located in the duodenal OSP (a–h) and ISP (i–p) of the pig. Merged images (d, h, l, p) are composites of images taken separately from red (a, c, i, m), green (b, f, j, n), and blue (c, g, k, o) fluorescent channels. Scale bar = 25 μm. a–d ZnT3+/VAChT−/NOS+—long arrows, ZnT3+/VAChT+/NOS−—small arrows; e–h ZnT3+/SP−/VIP+—long arrow, ZnT3+/SP+/VIP−—small arrows; i–l ZnT3+/VAChT+/SP+—long arrows, ZnT3+/VAChT+/SP−—small arrows; m–p ZnT3+/GAL+/VIP−—long arrows, ZnT3+/GAL−/VIP+—small arrows
Inner Submucosal Plexus
In the ISP, all ZnT3+ neurons were simultaneously cholinergic, i.e., ZnT3+/VAChT+ (Table 2 and Fig. 3i–j, l). The triple-labeling immunofluorescence revealed that among these neurons, 42.5 ± 1.2 % of cells were also GAL+, 36.6 ± 4.2 % were also VIP+, 26.2 ± 2.9 were also SP+, and 13.2 ± 3.8 coexpressed SOM (Table 2 and Fig. 3k, m–p). In addition, ZnT3+ neurons in the ISP were supplied with a very large number of VAChT+ nerve terminals and a moderate number of SOM+, SP+, and/or GAL+ nerve fibers (Fig. 3j–l, n–p). On the other hand, only a small number of VIP+ nerve fibers and single LENK+ and/or NOS+ nerve terminals were found around ZnT3+ neurons in the ISP.
Discussion
The present study for the first time demonstrates existence of the ZnT3+ neurons in the ENS of the porcine duodenum. The perikarya immunoreactive to ZnT3 have been found within all types of enteric ganglia, and these results are generally in agreement with previous investigations on the human and porcine large intestine (Gonkowski et al. 2007, 2009). According to the general knowledge about mammalian ENS, the neurons in the MP mainly participate in the regulation of gut motility (Brehmer et al. 1994; Huizinga et al. 2011) whereas the nerve cells within the OSP and ISP are taking part in excretory functions of the digestive tract (Brehmer et al. 2010). Thus, the considerable quantity of ZnT3-expressing cells, above 35 % of all perikarya in each plexus, may suggest that ZnT3 and Zn are important substances within the digestive tract, and they may be engaged at least in some of the gut's functions. Moreover, the previous study on the porcine large intestine (Gonkowski et al. 2007) indicates the participation of ZnT3 and Zn in mechanisms of pathological states within the digestive tract.
Although the precise function(s) of ZnT3 neurons and Zn is currently unknown in the enteric ganglia (this study and Gonkowski et al. 2007, 2009) and in the adrenergic and cholinergic sympathetic neurons of the murine peripheral nervous system (Wang et al. 2001a, 2003; Wang and Dahlstrom 2008), one could expect that ZnT3 and ionic zinc in the porcine duodenum may be, at least in part, implicated as a neurotransmitter/modulatory agent like within the CNS, where the functions of ZnT3 and Zn are better known (Cousins et al. 2006; Danscher et al. 2003; Kim et al. 2000; Palmiter and Huang 2004; Wang et al. 2003; Wenzel et al. 1997). It is generally accepted that ZnT3 plays an important role in the regulation of zinc levels, and it is the key protein in synaptic vesicle zinc transport (Danscher et al. 2003; Wenzel et al. 1997). Previous investigations on the CNS have suggested that ZnT3+ and Zn nervous structures may be involved in either the sensory transmission or the efferent control of other neuronal circuits (Danscher et al. 2001, 2003). Both ZnT3 and Zn are primarily present in glutamatergic terminals within the CNS (Danscher et al. 2001). However, Zn is also present in the GABA- and glycine-containing neurons (Danscher et al. 2001; Birinyi et al. 2001; Wang et al. 2001b). In the hippocampus, Zn is co-released with glutamate from ZEN synapses, both spontaneously and with electrical stimulation, where it exerts a strong modulatory effect on N-methyl-d-aspartate (NMDA) receptors (Vogt et al. 2000). Moreover, there is some convincing evidence to suggest that Zn may be a potent modulator of various receptors, ion channels, and several transporters in the brain, thereby influencing both excitatory and inhibitory neurotransmission (Betz and Laube 2006; Frederickson et al. 2005; Smart et al. 2004). Excitatory NMDA receptors are directly inhibited by Zn whereas non-NMDA receptors appear relatively unaffected (Smart et al. 2004; Paoletti and Neyton 2007). Because it is concentrated and released at many glutamatergic synapses, Zn is likely to be an endogenous allosteric modulator of the NMDA receptor (Paoletti and Neyton 2007). Inhibitory transmission mediated via GABA(A) receptors can be potentiated via a presynaptic mechanism, influencing transmitter release; however, although some tonic GABAergic inhibition may be suppressed by Zn, most synaptic GABA receptors are unlikely to be modulated directly by this cation (Smart et al. 2004). The glycinergic (inhibitory) transmission in the CNS may also be affected by Zn, thereby causing potentiation (Betz and Laube 2006). Low concentrations of Zn potentiate submaximal glycine-induced currents, whereas higher concentrations cause competitive inhibition (Betz and Laube 2006). A point mutation in the murine Glra1 locus, which selectively suppresses Zn potentiation, generates a phenotype that mimics that of patients with hyperekplexia (hereditary startle disease) and thus is indicative of decreased glycinergic inhibition (Betz and Laube 2006). It is worthy to note that cholinergic receptors in the brain may also be directly up- and downregulated by Zn while various other neurons may be modulated via other types of receptors like opioid or catecholamine receptors, various transporters, and several ion channels, including Ca2+, K+, or Cl− (Frederickson et al. 2005). These data, taken together, indicate that Zn is a potent modulator of both excitatory and inhibitory neurotransmission within the CNS. Therefore, it is tempting to speculate that it may have a similar role in the ENS. Such mechanism(s) may in part explain why so many excitatory cholinergic and inhibitory nitrergic intestinal neurons coexpress ZnT3 (present study).
Recent studies in the brain and other tissues indicate that ZnT3 and Zn may be implicated not only in neurotransmission but also in many other functions. For example, previous studies on Zn-containing neurons in the CNS suggest that ZnT3 may protect neurons from the cytotoxic action of zinc during pathological processes (Cousins et al. 2003, 2006; Kim et al. 2000; Palmiter and Huang 2004). The number of ZnT3-containing neurons was also elevated in the large intestine during inflammation and axotomy suggesting the existence of similar mechanisms in the ENS (Gonkowski et al. 2007). Observations in neurons suggest that exposure to elevated levels of Zn can be cytotoxic, within minutes (Yokoyama et al. 1986). Several studies in the gastrointestinal tract confirm that oxidant stress induces Zn elevation that can influence pathways of cell death and the balance between necrosis and apoptosis (Cima et al. 2006; Kohler et al. 2009, 2010). Alternatively, extracellular Zn acting via zinc-sensing receptors (ZnR) is able to activate major signaling pathways linked to cellular proliferation and survival (Hershfinkel et al. 2001; Cohen et al. 2012). For example, in the intestines, extracellular Zn, acting through ZnR, regulates intracellular pH and clusterin expression thereby enhancing survival of colonocytes (Cohen et al. 2012). Similar zinc sensors might be present in other tissues, including the neurons. Moreover, extracellular Zn is not the only player in the animal tissues. For example, Ca2+ facilitates Zn uptake, and oxidative dysregulation of intracellular Zn could be amplified by dysregulation of intracellular Ca2+ homeostasis (Liu et al. 2011). This potential relationship between Zn and Ca2+ levels may in part explain the role of Zn and Zn transporters in the intestinal secretion (Liu et al. 2011). Based on the well-recognized role of Ca2+ as a second messenger in responses to secretagogues (Caroppo et al. 2001; Perez-Zoghbi et al. 2008), there is a novel concept that Ca2+ may be also a second messenger that can match the basolateral demand for Zn with the secretory response to physiologic stimulation (Liu et al. 2011). Similar connections may be also present in other secretory cells including neural, endocrine, and exocrine tissues.
Among the possible ways to elucidate the functions of ZnT3 within the ENS are investigations on co-localization of this peptide with other better known active substances in the same cell bodies. It is generally accepted that each enteric neuron can contain several active substances. Moreover, immunohistochemical investigations on the ENS revealed that each functional class of enteric neurons respectively contains a unique combination of chemical markers (for review, Furness 2012). The present study has shown that ZnT3 in enteric neurons of the porcine duodenum were co-localized with various active substances such as VAChT, NOS, SOM, SP, GAL, or LENK, and the percentage of co-localization was dependent on the type of enteric plexus. Most of the ZnT3+ myenteric neurons were also immunoreactive to VAChT and/or NOS. These neurons are one of a number of elements that are responsible for the control of digestive motility (Huizinga et al. 2011), and a subpopulation of myenteric neurons also innervates the submucosal plexuses and/or regulates the secretory functions of the gut (Brehmer et al. 1994). VAChT is the histochemical marker of acetylcholine, and gut motility is controlled by a subpopulation of cholinergic and nitrergic neurons which mediate the respective contraction and relaxation of circular and longitudinal muscle (Brehmer et al. 2006; Boeckxstaens et al. 1993; Furness 2006; Lincoln et al. 1997; Porter et al. 1996, 1997; Wood et al. 1999; Qu et al. 2008). Since the majority of ZnT3+ myenteric neurons were also immunoreactive to VAChT and/or NOS, thus ZnT3 and Zn may probably modulate the activity of both neuron populations that act together in coordinated reflexes to facilitate gut smooth muscle contraction (VAChT neurons) and muscle relaxation (NOS neurons), respectively. The neurons of the OSP and ISP innervate the submucosal blood vessels and regulate the secretion and intrinsic sensory pathways of the gut in response to the contents of the lumen (Brehmer et al. 2010). A subpopulation of the OSP neurons can also supply the circular muscle layer of the gut (Scheuermann and Timmermans 1993). More than half of all ZnT3+ neurons located in the OSP and all these neurons in the ISP were simultaneously immunoreactive to VAChT. Such a large population of ZnT3+/VAChT+ neurons indicate that ZnT3 and Zn may be engaged in various excitatory functions in the OSP and ISP including muscle contraction in the muscularis mucosa and the increased activity of mucosal glands (Cooke 2000). VIP was coexpressed in ZnT3+ neurons of both submucosal plexuses but especially in the ISP. This is congruent with the fact that submucosal VIP neurons are responsible for increasing secretory activity at mucosal glands in the small intestine (Olsson and Holmgren 2001). GAL was highly coexpressed by ZnT3+ neurons in the ISP, while in the OSP, such cells were rare (almost half of ZnT3+ neurons in the ISP coexpressed GAL). GAL is often expressed in the ISP by neurons which are engaged in the regulation of the intestinal secretion (Furness 2006) and/or neurotransmitters secretion from other intestinal neurons (Piqueras et al. 2004; Sarnelli et al. 2004). Extensive co-localization of ZnT3 and GAL in the ISP neurons may further indicate that Zn and ZnT3 are involved in these processes of the gut.
In summary, the large number of ZnT3+ neurons in the ENS of the porcine duodenum and a broad spectrum of active substances which co-localize with this peptide as well as the previous studies on the porcine digestive tract (Gonkowski et al. 2007, 2009) suggest that ZnT3 is involved in the regulation of various processes in the gut, both in physiology and during pathological processes. By analogy to previous investigations on the central nervous system (Cousins et al. 2006; Danscher et al. 2003; Kim et al. 2000; Palmiter and Huang 2004; Wang et al. 2003; Wenzel et al. 1997), it is likely that ZnT3 is present in enteric neurons, which use zinc as neuromodulator, and/or it has a functional role that protects against the cytotoxic action of zinc. However, further studies are needed to elucidate the exact physiological role of ZnT3 within the enteric nervous system of the porcine GI tract.
References
Betz H, Laube B (2006) Glycine receptors: recent insights into their structural organization and functional diversity. J Neurochem 97:1600–1610
Birinyi A, Parker D, Antal M, Shupliakov O (2001) Zinc co-localizes with GABA and glycine in synapses in the lamprey spinal cord. J Comp Neurol 433:208–221
Boeckxstaens GE, Pelckmans PA, Herman AG, Van Maercke YM (1993) Involvement of nitric oxide in the inhibitory innervation of the human isolated colon. Gastroenterology 104:690–697
Brehmer A, Stach W, Addicks K (1994) Fine structural distinction between ganglia of the outer and inner submucosal plexus in porcine small intestine. Acta Anat (Basel) 151:188–193
Brehmer A, Schrodl F, Neuhuber W (2006) Morphology of VIP/nNOS-immunoreactive myenteric neurons in the human gut. Histochem Cell Biol 125:557–565
Brehmer A, Rupprecht H, Neuhuber W (2010) Two submucosal nerve plexus in human intestines. Histochem Cell Biol 133:149–161
Brookes SJ (2001) Classes of enteric nerve cells in the guinea-pig small intestine. Anat Rec 262:58–70
Brown DR, Timmermans JP (2004) Lessons from the porcine enteric nervous system. Neurogastroenterol Motil 16:50–54
Caroppo R, Gerbino A, Debellis L, Kifor O, Soybel DI, Brown EM, Hofer AM, Curci S (2001) Asymmetrical, agonist-induced fluctuations in local extracellular [Ca(2+)] in intact polarized epithelia. EMBO J 20:6316–6326
Cima RR, Dubach JM, Wieland AM, Walsh BM, Soybel DI (2006) Intracellular Ca(2+) and Zn(2+) signals during monochloramine-induced oxidative stress in isolated rat colon crypts. Am J Physiol Gastrointest Liver Physiol 290:G250–G261
Cohen L, Azriel-Tamir H, Arotsker N, Sekler I, Hershfinkel M (2012) Zinc sensing receptor signaling, mediated by GPR39, reduces butyrate-induced cell death in HT29 colonocytes via upregulation of clusterin. PLoS One 7:e35482
Cooke HJ (2000) Neurotransmitters in neuronal reflexes regulating intestinal secretion. Ann N Y Acad Sci 915:77–80
Costa M, Brookes SJ, Steele PA, Gibbins I, Burcher E, Kandiah CJ (1996) Neurochemical classification of myenteric neurons in the guinea-pig ileum. Neuroscience 75:949–967
Cousins RJ, Blanchard RK, Moore JB, Cui L, Green CL, Liuzzi JP, Cao J, Bobo JA (2003) Regulation of zinc metabolism and genomic outcomes. J Nutr 133:1521S–1526S
Cousins RJ, Liuzzi JP, Lichten LA (2006) Mammalian zinc transport, trafficking, and signals. J Biol Chem 281:24085–24089
Danscher G (1982) Exogenous selenium in the brain. A histochemical technique for light and electron microscopical localization of catalytic selenium bonds. Histochemistry 76:281–293
Danscher G, Jo SM, Varea E, Wang Z, Cole TB, Schroder HD (2001) Inhibitory zinc-enriched terminals in mouse spinal cord. Neuroscience 105:941–947
Danscher G, Wang Z, Kim YK, Kim SJ, Sun Y, Jo SM (2003) Immunocytochemical localization of zinc transporter 3 in the ependyma of the mouse spinal cord. Neurosci Lett 342:81–84
Frederickson CJ, Kasarskis EJ, Ringo D, Frederickson RE (1987) A quinoline fluorescence method for visualizing and assaying the histochemically reactive zinc (bouton zinc) in the brain. J Neurosci Methods 20:91–103
Frederickson CJ, Suh SW, Silva D, Frederickson CJ, Thompson RB (2000) Importance of zinc in the central nervous system: the zinc-containing neuron. J Nutr 130:1471S–1483S
Frederickson CJ, Koh JY, Bush AI (2005) The neurobiology of zinc in health and disease. Nat Rev Neurosci 6:449–462
Furness JB (2000) Types of neurons in the enteric nervous system. J Auton Nerv Syst 81:87–96
Furness JB (2006) The enteric nervous system. Blackwell, Oxford
Furness JB (2012) The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 9:286–294
Gaither LA, Eide DJ (2001) Eukaryotic zinc transporters and their regulation. Biometals 14:251–270
Gonkowski S, Bossowska A, Wojtkiewicz J, Skobowiat C, Majewski M (2007) Proliferative enteropathy (PE)-induced changes in the number of ZnT3-like immunoreactive (ZnT3-LI) colonic neurons in the pig. Anatomische Gesellschaft 102nd Annual Meeting in Giessen—Poster Abstract Booklet, p 47
Gonkowski S, Kaminska B, Landowski P, Skobowiat C, Burliński P, Majewski M, Calka J (2009) A population of zinc transporter 3 - like immunoreactivite neurons is present in the ganglia of human descending colon. Adv Clin Exp Med 18:243–248
Hershfinkel M, Moran A, Grossman N, Sekler I (2001) A zinc-sensing receptor triggers the release of intracellular Ca2+ and regulates ion transport. Proc Natl Acad Sci USA 98:11749–11754
Huizinga JD, Martz S, Gil V, Wang XY, Jimenez M, Parsons S (2011) Two independent networks of interstitial cells of cajal work cooperatively with the enteric nervous system to create colonic motor patterns. Front Neurosci 5:93
Jo SM, Danscher G, Daa SH, Won MH, Cole TB (2000) Zinc-enriched (ZEN) terminals in mouse spinal cord: immunohistochemistry and autometallography. Brain Res 870:163–169
Kim AH, Sheline CT, Tian M, Higashi T, McMahon RJ, Cousins RJ, Choi DW (2000) L-Type Ca(2+) channel-mediated Zn(2+) toxicity and modulation by ZnT-1 in PC12 cells. Brain Res 886:99–107
Kohler JE, Mathew J, Tai K, Blass AL, Kelly E, Soybel DI (2009) Monochloramine impairs caspase-3 through thiol oxidation and Zn2+ release. J Surg Res 153:121–127
Kohler JE, Dubach JM, Naik HB, Tai K, Blass AL, Soybel DI (2010) Monochloramine-induced toxicity and dysregulation of intracellular Zn2+ in parietal cells of rabbit gastric glands. Am J Physiol Gastrointest Liver Physiol 299:G170–G178
Lincoln J, Hoyle CH, Cafri G (1997) Nitric oxide in health and disease. Cambridge University Press, Cambridge
Liu J, Kohler JE, Blass AL, Moncaster JA, Mocofanescu A, Marcus MA, Blakely EA, Bjornstad KA, Amarasiriwardena C, Casey N, Goldstein LE, Soybel DI (2011) Demand for Zn2+ in acid-secreting gastric mucosa and its requirement for intracellular Ca2+. PLoS One 6:e19638
Olsson C, Holmgren S (2001) The control of gut motility. Comp Biochem Physiol A Mol Integr Physiol 128:481–503
Palmiter RD, Huang L (2004) Efflux and compartmentalization of zinc by members of the SLC30 family of solute carriers. Pflugers Arch 447:744–751
Paoletti P, Neyton J (2007) NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol 7:39–47
Perez-Zoghbi JF, Mayora A, Ruiz MC, Michelangeli F (2008) Heterogeneity of acid secretion induced by carbachol and histamine along the gastric gland axis and its relationship to [Ca2+]i. Am J Physiol Gastrointest Liver Physiol 295:G671–G681
Piqueras L, Tache Y, Martinez V (2004) Galanin inhibits gastric acid secretion through a somatostatin-independent mechanism in mice. Peptides 25:1287–1295
Porter AJ, Wattchow DA, Brookes SJ, Schemann M, Costa M (1996) Choline acetyltransferase immunoreactivity in the human small and large intestine. Gastroenterology 111:401–408
Porter AJ, Wattchow DA, Brookes SJ, Costa M (1997) The neurochemical coding and projections of circular muscle motor neurons in the human colon. Gastroenterology 113:1916–1923
Qu ZD, Thacker M, Castelucci P, Bagyanszki M, Epstein ML, Furness JB (2008) Immunohistochemical analysis of neuron types in the mouse small intestine. Cell Tissue Res 334:147–161
Sarnelli G, Vanden BP, Raeymaekers P, Janssens J, Tack J (2004) Inhibitory effects of galanin on evoked [Ca2+]i responses in cultured myenteric neurons. Am J Physiol Gastrointest Liver Physiol 286:G1009–G1014
Scheuermann DW, Timmermans JP (1993) Differing chemical content of the neuronal populations of submucosal ganglionic plexus of the enteric nervous system. Gastroenterology 104:1579
Smart TG, Hosie AM, Miller PS (2004) Zn2+ ions: modulators of excitatory and inhibitory synaptic activity. Neuroscientist 10:432–442
Vogt K, Mellor J, Tong G, Nicoll R (2000) The actions of synaptically released zinc at hippocampal mossy fiber synapses. Neuron 26:187–196
Wang ZY, Dahlstrom A (2008) Axonal transport of zinc transporter 3 and zinc containing organelles in the rodent adrenergic system. Neurochem Res 33:2472–2479
Wang Z, Danscher G, Mook JS, Shi Y, Daa SH (2001a) Retrograde tracing of zinc-enriched (ZEN) neuronal somata in rat spinal cord. Brain Res 900:80–87
Wang Z, Li JY, Dahlstrom A, Danscher G (2001b) Zinc-enriched GABAergic terminals in mouse spinal cord. Brain Res 921:165–172
Wang ZY, Danscher G, Dahlstrom A, Li JY (2003) Zinc transporter 3 and zinc ions in the rodent superior cervical ganglion neurons. Neuroscience 120:605–616
Wenzel HJ, Cole TB, Born DE, Schwartzkroin PA, Palmiter RD (1997) Ultrastructural localization of zinc transporter-3 (ZnT-3) to synaptic vesicle membranes within mossy fiber boutons in the hippocampus of mouse and monkey. Proc Natl Acad Sci USA 94:12676–12681
Wood JD, Alpers DH, Andrews PL (1999) Fundamentals of neurogastroenterology. Gut 45(Suppl 2):II6–II16
Yokoyama M, Koh J, Choi DW (1986) Brief exposure to zinc is toxic to cortical neurons. Neurosci Lett 71:351–355
Acknowledgments
This work was supported by grant no. NN401178639 from the State Committee for Science Research of Poland. The authors would like to thank Prof. Richard Palmiter from Howard Hughes Medical Institute and Department of Biochemistry, University of Washington, for donating the primary antibody (zinc transporter 3).
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Wojtkiewicz, J., Gonkowski, S., Równiak, M. et al. Neurochemical Characterization of Zinc Transporter 3-Like Immunoreactive (ZnT3+) Neurons in the Intramural Ganglia of the Porcine Duodenum. J Mol Neurosci 48, 766–776 (2012). https://doi.org/10.1007/s12031-012-9855-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-012-9855-9