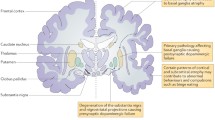
Abstract
Inclusion body myopathy associated with Paget’s disease of bone and frontotemporal dementia (IBMPFD) is a progressive, fatal genetic disorder with variable penetrance, predominantly affecting three main tissue types: muscle (IBM), bone (PDB), and brain (FTD). IBMPFD is caused by mutations in the ubiquitously expressed valosin-containing protein (VCP) gene, a member of the AAA-ATPase superfamily. The majority of individuals who develop IBM have progressive proximal muscle weakness. Muscle biopsies reveal rimmed vacuoles and inclusions that are ubiquitin- and TAR DNA binding protein-43 (TDP-43)-positive using immunohistochemistry. PDB, seen in half the individuals, is caused by overactive osteoclasts and is associated clinically with pain, elevated serum alkaline phosphatase, and X-ray findings of coarse trabeculation and sclerotic lesions. FTD diagnosed at a mean age of 55 years in a third of individuals is characterized clinically by comprehension deficits, dysnomia, dyscalculia, and social unawareness. Ubiquitin- and TDP-43-positive neuronal inclusions are also found in the brain. Genotype–phenotype correlations are difficult with marked intra-familial and inter-familial variations being seen. Varied phenotypes within families include frontotemporal dementia, amyotrophic lateral sclerosis, Parkinsonism, myotonia, cataracts, and anal incompetence, among others. Cellular and animal models indicate pathogenetic disturbances in IBMPFD tissues including altered protein degradation, autophagy pathway alterations, apoptosis, and mitochondrial dysfunction. Currently, mouse and drosophila models carrying VCP mutations provide insights into the human IBMPFD pathology and are useful as tools for preclinical studies and testing of therapeutic strategies. In this review, we will explore the pathogenesis and clinical phenotype of IBMPFD caused by VCP mutations.


Similar content being viewed by others
References
Acharyya S et al (2007) Interplay of IKK/NF-kappaB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J Clin Invest 117:889–901
Aggarwal BB (2004) Nuclear factor-kappaB: the enemy within. Cancer Cell 6:203–208
Alexandru G et al (2008) UBXD7 binds multiple ubiquitin ligases and implicates p97 in HIF1alpha turnover. Cell 134:804–816
Alonso A et al (2009) Incidence and lifetime risk of motor neuron disease in the United Kingdom: a population-based study. Eur J Neurol 16:745–751
Arnold SE et al (2000) Quantitative neurohistological features of frontotemporal degeneration. Neurobiol Aging 21:913–919
Badadani M et al (2010) VCP associated inclusion body myopathy and Paget disease of bone knock-in mouse model exhibits tissue pathology typical of human disease. PLoS One 5:1–15
Balch WE et al (2008) Adapting proteostasis for disease intervention. Science 319:916–919
Bersano A et al (2009) Inclusion body myopathy and frontotemporal dementia caused by a novel VCP mutation. Neurobiol Aging 30:752–758
Bhatnagar S, Kumar A (2010) Therapeutic targeting of signaling pathways in muscular dystrophy. J Mol Med 88:155–166
Cairns NJ et al (2007) TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol 171:227–240
Chang YC et al (2011) Pathogenic VCP/TER94 alleles are dominant actives and contribute to neurodegeneration by altering cellular ATP level in a Drosophila IBMPFD model. PLoS Genet 7:e1001288
Confalonieri F, Duguet M (1995) A 200-amino acid ATPase module in search of a basic function. Bioessays 17:639–650
Crippa V et al (2010a) A role of small heat shock protein B8 (HspB8) in the autophagic removal of misfolded proteins responsible for neurodegenerative diseases. Autophagy 6:958–960
Crippa V et al (2010b) The small heat shock protein B8 (HspB8) promotes autophagic removal of misfolded proteins involved in amyotrophic lateral sclerosis (ALS). Hum Mol Genet 19:3440–3456
Custer SK et al (2010) Transgenic mice expressing mutant forms VCP/p97 recapitulate the full spectrum of IBMPFD including degeneration in muscle, brain and bone. Hum Mol Genet 19:1741–1755
Dai RM et al (1998) Involvement of valosin-containing protein, an ATPase Co-purified with IkappaBalpha and 26 S proteasome, in ubiquitin–proteasome-mediated degradation of IkappaBalpha. J Biol Chem 273:3562–3573
Dai RM, Li CC (2001) Valosin-containing protein is a multi-ubiquitin chain-targeting factor required in ubiquitin-proteasome degradation. Nat Cell Biol 3:740–744
Daroszewska A, Ralston SH (2006) Mechanisms of disease: genetics of Paget's disease of bone and related disorders. Nat Clin Pract Rheumatol 2:270–277
DeLaBarre B et al (2006) Central pore residues mediate the p97/VCP activity required for ERAD. Mol Cell 22:451–462
Djamshidian A et al (2009) A novel mutation in the VCP gene (G157R) in a German family with inclusion-body myopathy with Paget disease of bone and frontotemporal dementia. Muscle Nerve 39:389–391
Fanganiello RD et al (2011) A Brazilian family with hereditary inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia. Braz J Med Biol Res 44:374–380
Farpour F et al (2011) Radiological features of Paget disease of bone associated with VCP myopathy. PMID: 21643886
Fernandez-Saiz V, Buchberger A (2010) Imbalances in p97 co-factor interactions in human proteinopathy. EMBO Rep 11:479–485
Gidaro T et al (2008) An Italian family with inclusion-body myopathy and frontotemporal dementia due to mutation in the VCP gene. Muscle Nerve 37:111–114
Graham KM et al (2010) Excessive collagen accumulation in dystrophic (mdx) respiratory musculature is independent of enhanced activation of the NF-kappaB pathway. J Neurol Sci 294:43–50
Guyant-Marechal L et al (2006) Valosin-containing protein gene mutations: clinical and neuropathologic features. Neurology 67:644–651
Haslbeck KM et al (2005) The RAGE pathway in inflammatory myopathies and limb girdle muscular dystrophy. Acta Neuropathol 110:247–254
Haubenberger D et al (2005) Inclusion body myopathy and Paget disease is linked to a novel mutation in the VCP gene. Neurology 65:1304–1305
Hayden MS, Ghosh S (2004) Signaling to NF-kappaB. Genes Dev 18:2195–2224
Hubbers CU et al (2007) Pathological consequences of VCP mutations on human striated muscle. Brain 130:381–393
Janiesch PC et al (2007) The ubiquitin-selective chaperone CDC-48/p97 links myosin assembly to human myopathy. Nat Cell Biol 9:379–390
Jarosch E et al (2002) Protein dislocation from the endoplasmic reticulum—pulling out the suspect. Traffic 3:530–536
Johnson JO et al (2010) Exome sequencing reveals VCP mutations as a cause of familial ALS. Neuron 68:857–864
Ju JS, Weihl CC (2010) Inclusion body myopathy, Paget's disease of the bone and fronto-temporal dementia: a disorder of autophagy. Hum Mol Genet 19:R38–R45
Kakizuka A (2008) Roles of VCP in human neurodegenerative disorders. Biochem Soc Trans 36:105–108
Kaleem M et al (2007) Identification of a novel valosin-containing protein polymorphism in late-onset Alzheimer's disease. Neurodegener Dis 4:376–381
Kim EJ, Park YE, Kim DS et al (2011) Inclusion body myopathy with Paget disease of bone and frontotemporal dementia linked to VCP p.Arg155Cys in a Korean family. Arch Neurol 68:787–796
Kimonis VE et al (2008a) VCP disease associated with myopathy, Paget disease of bone and frontotemporal dementia: review of a unique disorder. Biochim Biophys Acta 1782:744–748
Kimonis VE et al (2000) Clinical and molecular studies in a unique family with autosomal dominant limb-girdle muscular dystrophy and Paget disease of bone. Genet Med 2:232–241
Kimonis VE et al (2008b) Clinical studies in familial VCP myopathy associated with Paget disease of bone and frontotemporal dementia. Am J Med Genet A 146A:745–757
Kondo H et al (1997) p47 is a cofactor for p97-mediated membrane fusion. Nature 388:75–78
Korolchuk VI et al (2009) Autophagy inhibition compromises degradation of ubiquitin–proteasome pathway substrates. Mol Cell 33:517–527
Kovach MJ et al (2001) Clinical delineation and localization to chromosome 9p13.3-p12 of a unique dominant disorder in four families: hereditary inclusion body myopathy, Paget disease of bone, and frontotemporal dementia. Mol Genet Metab 74:458–475
Krause S et al (2007) Brain imaging and neuropsychology in late-onset dementia due to a novel mutation (R93C) of valosin-containing protein. Clin Neuropathol. 26:232–240
Kumar KR et al (2010) Two Australian families with inclusion-body myopathy, Paget's disease of bone and frontotemporal dementia: novel clinical and genetic findings. Neuromuscul Disord 20:330–334
Kwiatkowski TJ Jr et al (2009) Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 323:1205–1208
Lagier-Tourenne C et al (2010) TDP-43 and FUS/TLS: emerging roles in RNA processing and neurodegeneration. Hum Mol Genet 19:R46–R64
Leigh PN, Wijesekera LC (2010) Motor neuron disease: focusing the mind on ALS: updated practice parameters. Nat Rev Neurol 6:191–192
Leyton CE, Hodges JR (2010) Frontotemporal dementias: recent advances and current controversies. Ann Indian Acad Neurol 13:S74–S80
Ling SC et al (2010) ALS-associated mutations in TDP-43 increase its stability and promote TDP-43 complexes with FUS/TLS. Proc Natl Acad Sci U S A 107:13318–13323
Logroscino G et al (2008) Descriptive epidemiology of amyotrophic lateral sclerosis: new evidence and unsolved issues. J Neurol Neurosurg Psychiatry 79:6–11
Lomen-Hoerth C et al (2003) Are amyotrophic lateral sclerosis patients cognitively normal? Neurology 60:1094–1097
McGuire V et al (1996) Incidence of amyotrophic lateral sclerosis in three counties in western Washington state. Neurology 47:571–573
Meyer HH et al (2000) A complex of mammalian ufd1 and npl4 links the AAA-ATPase, p97, to ubiquitin and nuclear transport pathways. EMBO J 19:2181–2192
Miller TD et al (2009) Inclusion body myopathy with Paget disease and frontotemporal dementia (IBMPFD): clinical features including sphincter disturbance in a large pedigree. J Neurol Neurosurg Psychiatry 80:583–584
Mizushima N et al (2004) In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell 15:1101–1111
Muller JM et al (2007) Targeted deletion of p97 (VCP/CDC48) in mouse results in early embryonic lethality. Biochem Biophys Res Commun 354:459–465
Neary D et al (1998) Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51:1546–1554
Pasquali L et al (2010) The role of autophagy: what can be learned from the genetic forms of amyotrophic lateral sclerosis. CNS Neurol Disord Drug Targets 9:268–278
Rabinovich E et al (2002) AAA-ATPase p97/Cdc48p, a cytosolic chaperone required for endoplasmic reticulum-associated protein degradation. Mol Cell Biol 22:626–634
Rabouille C et al (1998) Syntaxin 5 is a common component of the NSF- and p97-mediated reassembly pathways of Golgi cisternae from mitotic Golgi fragments in vitro. Cell 92:603–610
Rape M et al (2001) Mobilization of processed, membrane-tethered SPT23 transcription factor by CDC48(UFD1/NPL4), a ubiquitin-selective chaperone. Cell 107:667–677
Reid IR et al (2005) Comparison of a single infusion of zoledronic acid with risedronate for Paget's disease. N Engl J Med 353:898–908
Ritson GP et al (2010) TDP-43 mediates degeneration in a novel Drosophila model of disease caused by mutations in VCP/p97. J Neurosci 30:7729–7739
Roberson ED (2011) Contemporary approaches to Alzheimer's disease and frontotemporal dementia. Methods Mol Biol 670:1–9
Rohrer JD, Warren JD, Reiman D et al (2011) A novel exon 2 I27V VCP variant is associated with dissimilar clinical syndromes. J Neurol 258:1494–1496
Rusten TE, Filimonenko M, Rodahl LM et al (2008) ESCRTing autophagic clearance of aggregating proteins. Autophagy 4:233–236
Schroder R et al (2005) Mutant valosin-containing protein causes a novel type of frontotemporal dementia. Ann Neurol 57:457–461
Shaw CE (2010) Capturing VCP: another molecular piece in the ALS jigsaw puzzle. Neuron 68:812–814
Siciliano G et al (2010) Clinical trials for neuroprotection in ALS. CNS Neurol Disord Drug Targets 9:305–313
Siris E et al (1996) Comparative study of alendronate versus etidronate for the treatment of Paget's disease of bone. J Clin Endocrinol Metab 81:961–967
Spina et al (2008) Frontotemporal dementia associated with a valosin-containing protein mutation: report of three families. The FASEB Journal 22:58.4 (Abstract)
Sreedharan J et al (2008) TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 319:1668–1672
Stojkovic T et al (2009) Clinical outcome in 19 French and Spanish patients with valosin-containing protein myopathy associated with Paget's disease of bone and frontotemporal dementia. Neuromuscul Disord 19:316–323
Strong MJ et al (2003) Cognitive impairment, frontotemporal dementia, and the motor neuron diseases. Ann Neurol 54(Suppl 5):S20–S23
Traynor BJ et al (1999) Incidence and prevalence of ALS in Ireland, 1995–1997: a population-based study. Neurology 52:504–509
Turner RS et al (1996) Clinical, neuroimaging, and pathologic features of progressive nonfluent aphasia. Ann Neurol 39:166–173
van der Zee J et al (2009) Clinical heterogeneity in 3 unrelated families linked to VCP p.Arg159His. Neurology 73:626–632
Vance C et al (2009) Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science 323:1208–1211
Vesa J et al (2009) Valosin containing protein associated inclusion body myopathy: abnormal vacuolization, autophagy and cell fusion in myoblasts. Neuromuscul Disord 19:766–772
Wang Q et al (2004) Molecular perspectives on p97-VCP: progress in understanding its structure and diverse biological functions. J Struct Biol 146:44–57
Watts GD et al (2003) Clinical and genetic heterogeneity in chromosome 9p associated hereditary inclusion body myopathy: exclusion of GNE and three other candidate genes. Neuromuscul Disord 13:559–567
Watts GD et al (2004) Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet 36:377–381
Watts GD et al (2007) Novel VCP mutations in inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia. Clin Genet 72:420–426
Weihl CC et al (2006) Inclusion body myopathy-associated mutations in p97/VCP impair endoplasmic reticulum-associated degradation. Hum Mol Genet 15:189–199
Weihl CC et al (2007) Transgenic expression of inclusion body myopathy associated mutant p97/VCP causes weakness and ubiquitinated protein inclusions in mice. Hum Mol Genet 16:919–928
Weihl CC et al (2009) Valosin-containing protein disease: inclusion body myopathy with Paget's disease of the bone and fronto-temporal dementia. Neuromuscul Disord 19:308–315
Wong E, Cuervo AM (2010) Autophagy gone awry in neurodegenerative diseases. Nat Neurosci 13:805–811
Ye Y et al (2001) The AAA ATPase Cdc48/p97 and its partners transport proteins from the ER into the cytosol. Nature 414:652–656
Zhukareva V et al (2001) Loss of brain tau defines novel sporadic and familial tauopathies with frontotemporal dementia. Ann Neurol 49:165–175
Acknowledgments
We would like to thank the families, providers, and our numerous collaborators for their contribution to this manuscript. We acknowledge funding by the NIH for grants AR050236 and AG025159, the Muscular Dystrophy Association, and UC Irvine Institute of Translational Science (ICTS). We thank Mehrdad Zoleikhaeian and Mariella Simon for their critical reading of the manuscript.
Conflict of Interest Statement
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nalbandian, A., Donkervoort, S., Dec, E. et al. The Multiple Faces of Valosin-Containing Protein-Associated Diseases: Inclusion Body Myopathy with Paget’s Disease of Bone, Frontotemporal Dementia, and Amyotrophic Lateral Sclerosis. J Mol Neurosci 45, 522–531 (2011). https://doi.org/10.1007/s12031-011-9627-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-011-9627-y