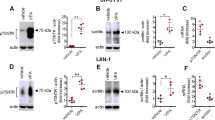
Abstract
Valproate, an anticonvulsant and mood stabilizer, up-regulates Bcl-2, a neurotrophic/neuroprotective protein. In this study, we investigated the molecular mechanism through which Bcl-2 is up-regulated by valproate using cultured human neuron-like cells. Valproate, within therapeutically relevant ranges, induced time- and concentration-dependent up-regulations of both Bcl-2 messenger RNA and protein implicating an underlying gene transcriptional-mediated mechanism. Bcl-2 up-regulations were associated with ERK1/2 and PI3K pathway activations and elevated levels of activated phospho-RSK and phospho-CREB, convergent targets of the ERK1/2 and PI3K pathways. Valproate increased transcriptional activity of a human bcl-2 promoter–reporter gene construct. This effect was attenuated, but not blocked, by mutation of a CREB DNA binding site, a CRE site in the human bcl-2 promoter sequence. ERK and/or PI3K pathway inhibitors and RSK1 small hairpin RNA knockdown reduced, but did not abolish, baseline and valproate-induced promoter activities and lowered Bcl-2 protein levels. These data collectively suggest that valproate induces Bcl-2 regulation partially through activations of the ERK and PI3K cascades and their convergent kinase, RSK, although other unknown mechanism(s) are likely involved. Given the known roles of Bcl-2 in the central nervous system, the current findings offer a partial yet complex molecular mechanistic explanation for the known neurobiological effects of valproate including neurite growth, neuronal survival, and neurogenesis.









Similar content being viewed by others
Abbreviations
- Bcl-2:
-
B-cell lymphoma 2
- BD:
-
bipolar (mood) disorder
- CRE:
-
cyclic adenosine monophosphate response element
- CREB:
-
cyclic adenosine monophosphate response element binding protein
- ERK:
-
extracellular signal-regulated kinase
- GSK-3:
-
glycogen synthase kinase-3
- HDAC:
-
histone deacetylase
- IMPase:
-
inositol monophosphatase
- PI3K:
-
phosphatidylinositol 3-kinase
- RSK:
-
p90 ribosomal S6 kinase
- VPA:
-
valproate
References
Almeida, R. D., Manadas, B. J., Melo, C. V., Gomes, J. R., Mendes, C. S., Graos, M. M., et al. (2005). Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death and Differentiation, 12, 1329–1343. doi:10.1038/sj.cdd.4401662.
Atack, J. R., Prior, A. M., Fletcher, S. R., Quirk, K., McKernan, R., & Ragan, C. I. (1994). Effects of L-690,488, a prodrug of the bisphosphonate inositol monophosphatase inhibitor L-690,330, on phosphatidylinositol cycle markers. The Journal of Pharmacology and Experimental Therapeutics, 270, 70–76.
Barnabe-Heider, F., & Miller, F. D. (2003). Endogenously produced neurotrophins regulate survival and differentiation of cortical progenitors via distinct signaling pathways. The Journal of Neuroscience, 23, 5149–5160.
Belmaker, R. H. (2004). Bipolar disorder. The New England Journal of Medicine, 351, 476–486. doi:10.1056/NEJMra035354.
Berridge, M. J., Downes, C. P., & Hanley, M. R. (1989). Neural and developmental actions of lithium: A unifying hypothesis. Cell, 59, 411–419. doi:10.1016/0092-8674(89)90026-3.
Chen, D. F., Schneider, G. E., Martinou, J. C., & Tonegawa, S. (1997). Bcl-2 promotes regeneration of severed axons in mammalian CNS. Nature, 385, 434–439. doi:10.1038/385434a0.
Chen, G., Huang, L. D., Jiang, Y. M., & Manji, H. K. (1999a). The mood-stabilizing agent valproate inhibits the activity of glycogen synthase kinase-3. Journal of Neurochemistry, 72, 1327–1330. doi:10.1046/j.1471-4159.2000.0721327.x.
Chen, G., Zeng, W. Z., Yuan, P. X., Huang, L. D., Jiang, Y. M., Zhao, Z. H., et al. (1999b). The mood-stabilizing agents lithium and valproate robustly increase the levels of the neuroprotective protein bcl-2 in the CNS. Journal of Neurochemistry, 72, 879–882. doi:10.1046/j.1471-4159.1999.720879.x.
Chen, P. S., Peng, G. S., Li, G., et al. (2006). Valproate protects dopaminergic neurons in midbrain neuron/glia cultures by stimulating the release of neurotrophic factors from astrocytes. Molecular Psychiatry, 11, 1116–1125. doi:10.1038/sj.mp.4001893.
Chuang, D. M. (2005). The antiapoptotic actions of mood stabilizers: Molecular mechanisms and therapeutic potentials. Annals of the New York Academy of Sciences, 1053, 195–204. doi:10.1196/annals.1344.018.
Dajas-Bailador, F. A., Soliakov, L., & Wonnacott, S. (2002). Nicotine activates the extracellular signal-regulated kinase 1/2 via the alpha7 nicotinic acetylcholine receptor and protein kinase A, in SH-SY5Y cells and hippocampal neurones. Journal of Neurochemistry, 80, 520–530. doi:10.1046/j.0022-3042.2001.00725.x.
De Mesquita, D. D., Zhan, Q., Crossley, L., & Badwey, J. A. (2001). p90-RSK and Akt may promote rapid phosphorylation/inactivation of glycogen synthase kinase 3 in chemoattractant-stimulated neutrophils. FEBS Letters, 502, 84–88. doi:10.1016/S0014-5793(01)02669-2.
De Sarno, P., Li, X., & Jope, R. S. (2002). Regulation of Akt and glycogen synthase kinase-3 beta phosphorylation by sodium valproate and lithium. Neuropharmacology, 43, 1158–1164. doi:10.1016/S0028-3908(02)00215-0.
Di Daniel, E., Cheng, L., Maycox, P. R., & Mudge, A. W. (2006). The common inositol-reversible effect of mood stabilizers on neurons does not involve GSK3 inhibition, myo-inositol-1-phosphate synthase or the sodium-dependent myo-inositol transporters. Molecular and Cellular Neuroscience, 32, 27–36.
Drevets, W. C. (2000). Neuroimaging studies of mood disorders. Biological Psychiatry, 48, 813–829. doi:10.1016/S0006-3223(00)01020-9.
Dwivedi, Y., Rizavi, H. S., Conley, R. R., & Pandey, G. N. (2006). ERK MAP kinase signaling in post-mortem brain of suicide subjects: Differential regulation of upstream Raf kinases Raf-1 and B-Raf. Molecular Psychiatry, 11, 86–98. doi:10.1038/sj.mp.4001744.
Einat, H., Yuan, P., Gould, T. D., Li, J., Du, J., Zhang, L., et al. (2003). The role of the extracellular signal-regulated kinase signaling pathway in mood modulation. The Journal of Neuroscience, 23, 7311–7316.
Encinas, M., Iglesias, M., Llecha, N., & Comella, J. X. (1999). Extracellular-regulated kinases and phosphatidylinositol 3-kinase are involved in brain-derived neurotrophic factor-mediated survival and neuritogenesis of the neuroblastoma cell line SH-SY5Y. Journal of Neurochemistry, 73, 1409–1421. doi:10.1046/j.1471-4159.1999.0731409.x.
Frodin, M., & Gammeltoft, S. (1999). Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Molecular and Cellular Endocrinology, 151, 65–77. doi:10.1016/S0303-7207(99)00061-1.
Fukumoto, T., Morinobu, S., Okamoto, Y., Kagaya, A., & Yamawaki, S. (2001). Chronic lithium treatment increases the expression of brain-derived neurotrophic factor in the rat brain. Psychopharmacology, 158, 100–106. doi:10.1007/s002130100871.
Gobbi, G., & Janiri, L. (2006). Sodium- and magnesium-valproate in vivo modulate glutamatergic and GABAergic synapses in the medial prefrontal cortex. Psychopharmacology, 185, 255–262. doi:10.1007/s00213-006-0317-3.
Hao, Y., Creson, T., Zhang, L., Li, P., Du, F., Yuan, P., et al. (2004). Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. The Journal of Neuroscience, 24, 6590–6599. doi:10.1523/JNEUROSCI.5747-03.2004.
Hsieh, J., Nakashima, K., Kuwabara, T., Mejia, E., & Gage, F. H. (2004). Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proceedings of the National Academy of Sciences of the USA, 101, 16659–16664. doi:10.1073/pnas.0407643101.
Keen, J. C., Yan, L., Mack, K. M., Pettit, C., Smith, D., Sharma, D., et al. (2003). A novel histone deacetylase inhibitor, scriptaid, enhances expression of functional estrogen receptor alpha (ER) in ER negative human breast cancer cells in combination with 5-aza 2¢-deoxycytidine. Breast Cancer Research and Treatment, 81, 177–186. doi:10.1023/A:1026146524737.
King, T. D., Gandy, J. C., & Bijur, G. N. (2006). The protein phosphatase-1/inhibitor-2 complex differentially regulates GSK3 dephosphorylation and increases sarcoplasmic/endoplasmic reticulum calcium ATPase 2 levels. Experimental Cell Research, 312, 3693–3700. doi:10.1016/j.yexcr.2006.08.010.
Kopnisky, K. L., Chalecka-Franaszek, E., Gonzalez-Zulueta, M., & Chuang, D. M. (2003). Chronic lithium treatment antagonizes glutamate-induced decrease of phosphorylated CREB in neurons via reducing protein phosphatase 1 and increasing MEK activities. Neuroscience, 116, 425–435. doi:10.1016/S0306-4522(02)00573-0.
Kuhn, H. G., Biebl, M., Wilhelm, D., Li, M., Friedlander, R. M., & Winkler, J. (2005). Increased generation of granule cells in adult Bcl-2-overexpressing mice: A role for cell death during continued hippocampal neurogenesis. The European Journal of Neuroscience, 22, 1907–1915. doi:10.1111/j.1460-9568.2005.04377.x.
Laeng, P., Pitts, R. L., Lemire, A. L., Drabik, C. E., Weiner, A., Tang, H., et al. (2004). The mood stabilizer valproic acid stimulates GABA neurogenesis from rat forebrain stem cells. Journal of Neurochemistry, 91, 238–251. doi:10.1111/j.1471-4159.2004.02725.x.
Lai, J. S., Zhao, C., Warsh, J. J., & Li, P. P. (2006). Cytoprotection by lithium and valproate varies between cell types and cellular stresses. European Journal of Pharmacology, 539, 18–26. doi:10.1016/j.ejphar.2006.03.076.
Leinninger, G. M., Backus, C., Uhler, M. D., Lentz, S. I., & Feldman, E. L. (2004). Phosphatidylinositol 3-kinase and Akt effectors mediate insulin-like growth factor-I neuroprotection in dorsal root ganglia neurons. The FASEB Journal, 18, 1544–1546.
Liu, Y. Z., Boxer, L. M., & Latchman, D. S. (1999). Activation of the Bcl-2 promoter by nerve growth factor is mediated by the p42/p44 MAPK cascade. Nucleic Acids Research, 27, 2086–2090. doi:10.1093/nar/27.10.2086.
Mao, L. M., Tang, Q., & Wang, J. Q. (2007). Protein kinase C-regulated cAMP response element-binding protein phosphorylation in cultured rat striatal neurons. Brain Research Bulletin, 72, 302–308. doi:10.1016/j.brainresbull.2007.01.009.
Mayr, B., & Montminy, M. (2001). Transcriptional regulation by the phosphorylation-dependent factor CREB. Nature reviews, 2, 599–609.
Michaelis, M., Suhan, T., Michaelis, U. R., et al. (2006). Valproic acid induces extracellular signal-regulated kinase 1/2 activation and inhibits apoptosis in endothelial cells. Cell Death and Differentiation, 13, 446–453. doi:10.1038/sj.cdd.4401759.
Ozaki, N., & Chuang, D. M. (1997). Lithium increases transcription factor binding to AP-1 and cyclic AMP-responsive element in cultured neurons and rat brain. Journal of Neurochemistry, 69, 2336–2344.
Park, S. Y., Lee, J. Y., Choi, J. Y., Park, M. J., & Kim, D. S. (2006). Nerve growth factor activates brain-derived neurotrophic factor promoter IV via extracellular signal-regulated protein kinase 1/2 in PC12 cells. Molecules and Cells, 21, 237–243.
Phiel, C. J., Zhang, F., Huang, E. Y., Guenther, M. G., Lazar, M. A., & Klein, P. S. (2001). Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. The Journal of Biological Chemistry, 276, 36734–36741. doi:10.1074/jbc.M101287200.
Phrolov, K., Applebaum, J., Levine, J., Miodovnick, H., & Belmaker, R. H. (2004). Single-dose intravenous valproate in acute mania. The Journal of Clinical Psychiatry, 65, 68–70.
Rajkowska, G. (2002). Cell pathology in bipolar disorder. Bipolar Disorders, 4, 105–116. doi:10.1034/j.1399-5618.2002.01149.x.
Riccio, A., Ahn, S., Davenport, C. M., Blendy, J. A., & Ginty, D. D. (1999). Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science, 286, 2358–2361. doi:10.1126/science.286.5448.2358.
Richards, S. A., Fu, J., Romanelli, A., Shimamura, A., & Blenis, J. (1999). Ribosomal S6 kinase 1 (RSK1) activation requires signals dependent on and independent of the MAP kinase ERK. Current Biology, 9, 810–820. doi:10.1016/S0960-9822(99)80364-9.
Saini, H. S., Gorse, K. M., Boxer, L. M., & Sato-Bigbee, C. (2004). Neurotrophin-3 and a CREB-mediated signaling pathway regulate Bcl-2 expression in oligodendrocyte progenitor cells. Journal of Neurochemistry, 89, 951–961. doi:10.1111/j.1471-4159.2004.02365.x.
Shacka, J. J., & Roth, K. A. (2005). Regulation of neuronal cell death and neurodegeneration by members of the Bcl-2 family: Therapeutic implications. Current Drug Targets. CNS and Neurological Disorders, 4, 25–39. doi:10.2174/1568007053005127.
Shaltiel, G., Shamir, A., Shapiro, J., et al. (2004). Valproate decreases inositol biosynthesis. Biological Psychiatry, 56, 868–874. doi:10.1016/j.biopsych.2004.08.027.
Shi, Y., Vaden, D. L., Ju, S., Ding, D., Geiger, J. H., & Greenberg, M. L. (2005). Genetic perturbation of glycolysis results in inhibition of de novo inositol biosynthesis. The Journal of Biological Chemistry, 280, 41805–41810. doi:10.1074/jbc.M505181200.
Sugai, F., Yamamoto, Y., Miyaguchi, K., Zhou, Z., Sumi, H., Hamasaki, T., et al. (2004). Benefit of valproic acid in suppressing disease progression of ALS model mice. The European Journal of Neuroscience, 20, 3179–3183. doi:10.1111/j.1460-9568.2004.03765.x.
Wilson, B. E., Mochon, E., & Boxer, L. M. (1996). Induction of bcl-2 expression by phosphorylated CREB proteins during B-cell activation and rescue from apoptosis. Molecular and Cellular Biology, 16, 5546–5556.
Xiang, H., Wang, J., & Boxer, L. M. (2006). Role of the cyclic AMP response element in the bcl-2 promoter in the regulation of endogenous Bcl-2 expression and apoptosis in murine B cells. Molecular and Cellular Biology, 26, 8599–8606. doi:10.1128/MCB.01062-06.
Xing, J., Kornhauser, J. M., Xia, Z., Thiele, E. A., & Greenberg, M. E. (1998). Nerve growth factor activates extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways to stimulate CREB serine 133 phosphorylation. Molecular and Cellular Biology, 18, 1946–1955.
York, R. D., Molliver, D. C., Grewal, S. S., Stenberg, P. E., McCleskey, E. W., & Stork, P. J. (2000). Role of phosphoinositide 3-kinase and endocytosis in nerve growth factor-induced extracellular signal-regulated kinase activation via Ras and Rap1. Molecular and Cellular Biology, 20, 8069–8083. doi:10.1128/MCB.20.21.8069-8083.2000.
Yuan, P. X., Huang, L. D., Jiang, Y. M., Gutkind, J. S., Manji, H. K., & Chen, G. (2001). The mood stabilizer valproic acid activates mitogen-activated protein kinases and promotes neurite growth. The Journal of Biological Chemistry, 276, 31674–31683. doi:10.1074/jbc.M104309200.
Acknowledgments
This work was supported by the NIMH intramural program (Manji and Chen) and a NARSAD research award (Chen).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Creson, T.K., Yuan, P., Manji, H.K. et al. Evidence for Involvement of ERK, PI3K, and RSK in Induction of Bcl-2 by Valproate. J Mol Neurosci 37, 123–134 (2009). https://doi.org/10.1007/s12031-008-9122-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-008-9122-2