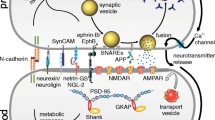
Abstract
The cellular prion protein (PrPC) is found prominently at the synapse. However, its role at the nerve termini and elsewhere is unknown. Here we discuss research presented at the 2005 International Institute for Complex Adaptive Matter (I2CAM) first Annual Amyloid Conference that provides insight into the role of synaptic PrPC. The prion protein can interact and facilitate copper uptake at the synapse, is presumed to oligodimerize to facilitate putative cell-cell adhesion, and it transports toward the synapse by fast microtubule-based anterograde transport. While PrPC appears to be involved in all these processes, the mechanisms of PrPC function in each of them remain unclear. A role for PrPC in these distinct processes suggests a complex role for this protein at the synapse. Unraveling PrPC function will likely entail employing combined approaches that take into account its possible multifaceted functions.
Similar content being viewed by others
References
Aplin, A. E., Howe, A., Alahari, S. K., & Juliano, R. L. (1998). Signal transduction and signal modulation by cell adhesion receptors: The role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacological Reviews, 50, 197–263.
Bailly, Y., Haeberle, A. M., Blanquet-Grossard, F., Chasserot-Golaz, S., Grant, N., Schulze, T., et al. (2004). Prion protein (PrPC) immunocytochemistry and expression of the green fluorescent protein reporter gene under control of the bovine PrP gene promoter in the mouse brain. Journal of Comparative Neurology, 473, 244–269.
Baldwin, M. A. (2005). Analysis of glycosylphosphatidylinositol protein anchors: The prion protein. Methods in Enzymology, 405, 172–187.
Baloui, H., von Boxberg, Y., Vinh, J., Weiss, S., Rossier, J., Nothias, F., et al. (2004). Cellular prion protein/laminin receptor: Distribution in adult central nervous system and characterization of an isoform associated with a subtype of cortical neurons. European Journal of Neuroscience, 20, 2605–2616.
Bendheim, P. E., Brown, H. R., Rudelli, R. D., Scala, L. J., Goller, N. L., Wen, G. Y., et al. (1992). Nearly ubiquitous tissue distribution of the scrapie agent precursor protein. Neurology, 42, 149–156.
Brown, D. R. (1999). Prion protein expression aids cellular uptake and veratridine-induced release of copper. Journal of Neuroscience Research, 58, 712–717
Brown, D. R., Qin, K., Herms, J. W., Madlung, A., Manson, J., Strome, R., et al. (1997). The cellular prion protein binds copper in vivo. Nature, 390, 684–687.
Buckley, C. D., Rainger, G. E., Brad¢eld, P. F., Nash, G. B., & Simmons, D. L. (1998). Cell adhesion: More than just glue. Molecular Membrane Biology, 15, 167–176.
Chishti, M. A., Strome, R., Carlson, G. A., & Westaway, D. (1997). Syrian hamster prion protein (PrP(C)) is expressed in photoreceptor cells of the adult retina. Neuroscience Letters, 234, 11–14.
Collinge, J., Whittington, M. A., Sidle, K. C., Smith, C. J., Palmer, M. S., Clarke, A. R., et al. (1994). Prion protein is necessary for normal synaptic function. Nature, 370, 295–297.
DeArmond, S. J., Mobley, W. C., DeMott, D. L., Barry, R. A., Beckstead, J. H., & Prusiner, S. B. (1987). Changes in the localization of brain prion proteins during scrapie infection. Neurology, 37, 1271–1280.
Ford, M. J., Burton, K. J., Morris, R. J., & Hall, S. M. (2002). Selective expression of prion protein in peripheral tissues of the adult mouse. Neuroscience, 113, 177–192.
Fournier, J.-G., Escaig-Haye, F., Billette de Villemeur, T., & Robain, O. (1995). Ultrastructural localization of cellular prion protein (PrPC) in synaptic boutons of normal hamster hippocampus. Comptes Rendus de l'AcadeÂmie des Sciences. Paris, III 318, 339–344.
Gabizon, R., Meiner, Z., Halimi, M., & Ben-Sasson, S. A. (1993). Heparin-like molecules bind differentially to prion-proteins and change their intracellular metabolic fate. Journal of Cellular Physiology, 157, 319–325.
Gauczynski, S., Peyrin, J. M., Haik, S., Leucht, C., Hundt, C., Rieger, R., et al. (2001). The 37-kDa/67-kDa laminin receptor acts as the cell-surface receptor for the cellular prion protein. EMBO Journal, 20, 5863–5875.
Guzik, B. W., & Goldstein, L. S. (2004). Microtubule-dependent transport in neurons: Steps towards an understanding of regulation, function and dysfunction. Current Opinion in Cell Biology, 16, 443–450.
Harris, D. A., Peters, P. J., Taraboulos, A., Lingappa, V., DeArmond, S. J., & Prusiner, S. B. (2003). Cell biology of prions. In S. B. Prusiner (Ed.), Prion biology and diseases (2nd ed., pp. 483–544).
Herms, J., Tings, T., Gall, S., Madlung, A., Giese, A., Siebert, H., et al. (1999). Evidence of presynaptic location and function of the prion protein. Journal of Neuroscience, 19, 8866–8875.
Hirokawa, N., & Takemura, R. (2005). Molecular motors and mechanisms of directional transport in neurons. Nature Reviews. Neuroscience, 6, 201–214.
Hornshaw, M. P., McDermott, J. R., Candy, J. M., & Lakey, J. H. (1995). Copper binding to the N-terminal tandem repeat region of mammalian and avian prion protein: Structural studies using synthetic peptides. Biochemical and Biophysical Research Communications, 214, 993–999.
Hundt, C., Gauczynski, S., Leucht, C., Riley, M. L., & Weiss, S. (2003). Intra- and interspecies interactions between prion proteins and effects of mutations and polymorphisms. Biological Chemistry, 384, 791–803.
Hundt, C., Peyrin, J. M., Haik, S., Gauczynski, S., Leucht, C., Rieger, R., et al. (2001). Identification of interaction domains of the prion protein with its 37-kDa/67-kDa laminin receptor. EMBO Journal, 20, 5876–5886.
Kretzschmar, H. A., Tings, T., Madlung, A., Giese, A., & Herms, J. (2000). Function of PrPC as a copper-binding protein at the synapse. Archives of Virology Supplementum, 16, 239–249.
Laine, J., Marc, M. E., Sy, M. S., & Axelrad, H. (2001). Cellular and subcellular morphological localization of normal prion protein in rodent cerebellum. European Journal of Neuroscience, 14, 47–56.
Lindquist, S. L. (2002–2003). Prion proteins: One surprise after another. Harvey Lectures, 98, 173–205.
Lopez Garcia, F., Zahn, R., Riek, R., & Wuthrich, K. (2000). NMR structure of the bovine prion protein. Proceedings of the National Academy of Sciences of the United States of America, 97, 8334–8339.
Mange, A., Milhavet, O., Umlauf, D., Harris, D., & Lehmann, S. (2002). PrP-dependent cell adhesion in N2a neuroblastoma cells. FEBS Letters, 514, 159–162.
Mironov, Jr., A., Latawiec, D., Wille, H., Bouzamondo-Bernstein, E., Legname, G., Williamson, R. A., et al. (2003). Cytosolic prion protein in neurons. Journal of Neuroscience, 23, 7183–7193.
Mouillet-Richard, S., Ermonval, M., Chebassier, C., Laplanche, J. L., Lehmann, S., Launay, J. M., et al. (2000). Signal transduction through prion protein. Science, 289, 1925–1928.
Moya, K. L., Hässig, R., Breen, K. C., Volland, H., & Di Giamberardino, L. (2005). Axonal transport of the cellular prion protein is increased during axon regeneration. Journal of Neurochemistry, 92, 1044–1053.
Moya, K. L., Hässig, R., Créminon, C., Laffont, I., & Di Giamberardino, L. (2004). Enhanced detection and retrograde axonal transport of PrPC in peripheral nerve. Journal of Neurochemistry, 88, 155–160.
Pauly, P. C., & Harris, D. A. (1998). Copper stimulates endocytosis of the prion protein. Journal of Biological Chemistry, 273, 33107–3310.
Perera, W. S., & Hooper, N. M. (2001). Ablation of the metal ion-induced endocytosis of the prion protein by disease-associated mutation of the octarepeat region. Current Biology, 11, 519–523.
Piccardo, P., Safar, J., Ceroni, M., Gajdusek, D. C., & Gibbs Jr., C. J. (1990). Immunohistochemical localization of prion protein in spongiform encephalopathies and normal brain tissue. Neurology, 40, 518–522.
Rachidi, W., Mange, A., Senator, A., Guiraud, P., Riondel, J., Benboubetra, M., et al. (2003b). Prion infection impairs copper binding of cultured cells. Journal of Biological Chemistry, 278, 14595–14598 (Epub 2003 Mar 10).
Rachidi, W., Vilette, D., Guiraud, P., Arlotto, M., Riondel, J., Laude, H., et al. (2003a). Expression of prion protein increases cellular copper binding and antioxidant enzyme activities but not copper delivery. Journal of Biological Chemistry, 278, 9064–9072 (Epub 2002 Dec 23).
Rahman, A., Friedman, D. S., & Goldstein, L. S. (1998). Two kinesin light chain genes in mice. Identification and characterization of the encoded proteins. Journal of Biological Chemistry, 273, 15395–15403.
Riek, R., Hornemann, S., Wider, G., Glockshuber, R., & Wuthrich, K. (1997). NMR characterization of the full-length recombinant murine prion protein, mPrP(23–231). FEBS Letters, 413, 282–288.
Rodolfo, K., Hässig, R., Moya, K. L., Frobert, Y., Grassi, J., & Di Giamberardino, L. (1999). A novel cellular prion protein isoform present in rapid anterograde axonal transport. NeuroReport, 10, 3639–3644.
Roucou, X., Gains, M., & LeBlanc, A. C. (2004). Neuroprotective functions of prion protein. Journal of Neuroscience Research, 75, 153–161.
Safar, J., Ceroni, M., Piccardo, P., Liberski, P. P., Miyazaki, M., Gajdusek, D. C., et al. (1990). Subcellular distribution and physicochemical properties of scrapie-associated precursor protein and relationship with scrapie agent. Neurology, 40, 503–508.
Sáles, N., Hassig, R., Rodolfo, K., Di Giamberardino, L., Traiffort, E., Ruat, M., et al. (2002). Developmental expression of the cellular prion protein in elongating axons. European Journal of Neuroscience, 15, 1163–1177.
Sáles, N., Rodolfo, K., Hassig, R., Faucheux, B., Di Giamberardino, L., & Moya, K. L. (1998). Cellular prion protein localization in rodent and primate brain. European Journal of Neuroscience, 10, 2464–2471.
Santuccione, A., Sytnyk, V., Leshchyns’ka, I., & Schachner, M. (2005). Prion protein recruits its neuronal receptor NCAM to lipid rafts to activate p59fyn and to enhance neurite outgrowth. Journal of Cell Biology, 169, 341–354.
Schachner, M., & Martini, R. (1995). Glycans and the modulation of neural-recognition molecule function. Proceedings of the National Academy of Sciences of the United States of America, 84, 6934–6938.
Shorter, J., Lindquist, S. (2005). Prions as adaptive conduits of memory and inheritance. Nature Reviews. Genetics, 6, 435–450.
Si, K., Lindquist, S., & Kandel, E. R. (2003). A neuronal isoform of the aplysia CPEB has prion-like properties. Cell, 115, 879–891.
Stahl, N., Borchelt, D. R., Hsiao, K., & Prusiner, S. B. (1987). Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell, 51, 229–240.
Vassallo, N., & Herms, J. (2003). Cellular prion protein function in copper homeostasis and redox signalling at the synapse. Journal of Neurochemistry, 86, 538–544.
Voshol, H., van Zuylen, C. W. E. M., Orberger, G., Vliegenthart, J. F. G., & Schachner, M. (1996). Structure of the HNK-1 carbohydrate epitope on bovine peripheral myelin glycoprotein P0. Journal of Biological Chemistry, 38, 22957–22960.
Warner, R. G., Hundt, C., Weiss, S., & Turnbull, J. E. (2002). Identification of the heparan sulfate binding sites in the cellular prion protein. Journal of Biological Chemistry, 277, 18421–18430.
Wickner, R. B., Edskes, H. K., Ross, E. D., Pierce, M. M., Shewmaker, F., Baxa, U., et al. (2004). Prions of yeast are genes made of protein: Amyloids and enzymes. Cold Spring Harbor Symposia on Quantitative Biology, 69, 489–496.
Wong, B. S., Chen, S. G., Colucci, M., Xie, Z., Pan, T., Liu, T., et al. (2001). Aberrant metal binding by prion protein in human prion disease. Journal of Neurochemistry, 78, 1400–1408.
Zahn, R., Liu, A., Luhrs, T., Riek, R., von Schroetter, C., Lopez Garcia, F., et al. (2000). NMR solution structure of the human prion protein. Proceedings of the National Academy of Sciences of the United States of America, 97, 145–150.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Encalada, S.E., Moya, K.L., Lehmann, S. et al. The Role of the Prion Protein in the Molecular Basis for Synaptic Plasticity and Nervous System Development. J Mol Neurosci 34, 9–15 (2008). https://doi.org/10.1007/s12031-007-0011-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-007-0011-x