Abstract
Introduction
It has been shown that CD44 may be associated with poor prognosis in various human malignancies. This study was designed to investigate and compare the prognostic relevance and clinical value of soluble forms of CD44 and its variant v6 (CD44v6) and their tissue expression in colorectal carcinoma.
Materials and methods
Serum levels of pre- and postoperative CD44 and CD44v6 molecules were evaluated in 37 colorectal cancer patients and compared to healthy individuals.
Results and discussion
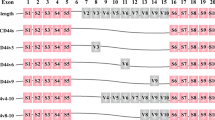
The serum levels of soluble CD44 and CD44v6 showed no significant decrease after surgical resection of the tumor (p = 0.5). Both CD44 and CD44v6 serum levels either before or after surgery were significantly higher in patients than in normal individuals (p < 0.001). The serum level of CD44v6 molecule was higher in patients with lymph node metastasis than other patients (p = 0.05) implying the role of this molecule in tumor progression. Immunohistochemical staining showed expression of CD44 in 14.3% and CD44v6 in 42.9% of the tumor samples. The expression of CD44v6 was associated with metastatic involvement of lymph nodes (p = 0.03). CD44v6 expression was positively correlated with its level in the serum of patients (p = 0.04).
Conclusions
Results of this study showed that CD44v6 expression level either in the soluble form or in the cell membrane is associated with tumor metastasis indicating the importance of this molecule in progression of colorectal carcinoma. The serum levels of soluble CD44 as well as CD44v6 might be useful markers for tumor screening.


Similar content being viewed by others
References
Hosseini SV, Izadpanah A, Yarmohammadi H. Epidemiological changes in colorectal cancer in Shiraz, Iran:1980–2000. ANZ J Surg. 2004;74:547–9.
Orr FW, Wang HH. Tumor cell interactions with the microvasculature: a rate-limiting step in metastasis. Surg Oncol Clin N Am. 2001;10:357–81.
Iiizumi M, Mohinta S, Bandyopadhyay S, et al. Tumor–endothelial cell interactions: therapeutic potential. Microvasc Res. 2007;74:114–20.
Dalchau R, Kirkley J, Fabre JW. Monoclonal anti-body to a human leukocyte-specific membrane glyco-protein probably homologous to the leukocyte-common (L-C) antigen of the rat. Eur J Immunol. 1980;10:737–44.
Dianzani U, Malavasi F. Lymphocyte adhesion to endothelium. Crit Rev Immunol. 1995;15:167–200.
Screaton GR, Bell MV, Jackson DG, et al. Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc Natl Acad Sci USA. 1992;89:12160–4.
Günthert U, Hofmann M, Rudy W, et al. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991;65:13–24.
Iida N, Bourguignon LYW. New CD44 splice variants associated with human breast cancers. J Cell Physiol. 1995;162:127–33.
Manten-Horst E, Danen EH, Smit L, et al. Expression of CD44 splice variants in human cutaneous melanoma and melanoma cell lines is related to tumor progression and metastatic potential. Int J Cancer Pred Oncol. 1995;64:182–8.
Picker LJ, De Los Toyos J, Telen MJ, et al. Monoclonal antibodies against the CD44 [in(lu)-related p80] and pgp-1 antigens in man recognize the Hermes class of lymphocyte homing receptors. J Immunol. 1989;142:2046–51.
Bazil V, Horejsi B. Shedding of the CD44 adhesion molecule from leukocytes induced by anti-CD44 monoclonal antibody simulating the effect of a natural receptor ligand. J Immunol. 1992;149:747–53.
Ristamäki R, Joensuu H, Jalkanen S. Does soluble CD44 reflect the clinical behavior of human cancer? Curr Top Microbiol Immunol. 1996;213:155–66.
Gansauge F, Gansauge S, Rau B, et al. Low serum levels of soluble CD44 variant 6 are significantly associated with poor prognosis in patients with pancreatic carcinoma. Cancer. 1997;80:1733–9.
Subramaniam V, Gardner H, Jothy S. Soluble CD44 secretion contributes to the acquisition of aggressive tumor phenotype in human colon cancer cells. Exp Molecular Pathol. 2007;83:341–6.
Cichy J, Pure E. The liberation of CD44. J Cell Biol. 2003;161:839–43.
Andratschke M, Chaubal S, Pauli C, et al. Soluble CD44v6 is not a sensitive tumor marker in patients with head and neck squamous cell cancer. Anticancer Res. 2005;25:2821–6.
Lein M, Jung K, Weiss S, et al. Soluble CD44 variants in the serum of patients with urological malignancies. Oncology. 1997;54:226–30.
Kainz C, Tempfer C, Winkler S, et al. Serum CD44 splice variants in cervical cancer patients. Cancer Lett. 1995;90:231–4.
Jung K, Lein M, Weiss S, et al. Soluble CD44 molecules in serum of patients with prostate cancer and benign prostatic hyperplasia. Eur J Cancer. 1996;32A:627–30.
Masson D, Denis MG, Denis M, et al. Soluble CD44: quantification and molecular repartition in plasma of patients with colorectal cancer. Br J Cancer. 1999;80:1995–2000.
Zalewski B. Levels of v5 and v6 CD44 splice variants in serum of patients with colorectal cancer are not correlated with pT stage, histopathological grade of malignancy and clinical features. World J Gastroenterol. 2004;10:583–5.
Weg-Remers S, Hildebrandt U, Feifel G, et al. Soluble CD44 and CD44v6 serum levels in patients with colorectal cancer are independent of tumor stage and tissue expression of CD44v6. Am J Gastroenterol. 1998;93:790–4.
Yamane N, Tsujitani S, Makino M, et al. Soluble CD44 variant 6 as a prognostic indicator in patients with colorectal Cancer. Oncology. 1999;56:232–8.
Perez-Palma J, Marchena-Gomez J, Dorta-Espineira M, et al. Predictive factors of years of potential life lost by colorectal cancer. Eur J Gastroenterol Hepatol. 2008;20:766–72.
Mai SK, Welzel G, Hermann B, et al. Long-term outcome after combined radiochemotherapy for anal cancer—retrospective analysis of efficacy, prognostic factors, and toxicity. Onkologie. 2008;31:251–7.
Bendardaf R, Algars A, Elzagheid A, et al. Comparison of CD44 expression in primary tumours and metastases of colorectal cancer. Oncol Rep. 2006;16:741–6.
Zhang JC, Wang ZR, Cheng YJ, et al. Expression of proliferating cell nuclear antigen and CD44 variant exon 6 in primary tumors and corresponding lymph node metastases of colorectal carcinoma with Dukes’ stage C or D. World J Gastroenterol. 2003;9:1482–6.
Acknowledgement
The authors are grateful to Fatemeh Safaei, Saied Malekhosseini, and Hossein Golmoghaddam for providing invaluable assistance. This work was supported by grants 2287 and 2802 from Shiraz University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amirghofran, Z., Jalali, S.A., Hosseini, S.V. et al. Evaluation of CD44 and CD44v6 in Colorectal Carcinoma Patients: Soluble Forms in Relation to Tumor Tissue Expression and Metastasis. J Gastrointest Canc 39, 73–78 (2008). https://doi.org/10.1007/s12029-009-9062-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-009-9062-2