Abstract
Background
Despite application of the multimodal European Resuscitation Council and European Society of Intensive Care Medicine algorithm, neurological prognosis of patients who remain comatose after cardiac arrest remains uncertain in a large group of patients. In this study, we investigate the additional predictive value of visual and quantitative brain magnetic resonance imaging (MRI) to electroencephalography (EEG) for outcome estimation of comatose patients after cardiac arrest.
Methods
We performed a prospective multicenter cohort study in patients after cardiac arrest submitted in a comatose state to the intensive care unit of two Dutch hospitals. Continuous EEG was recorded during the first 3 days and MRI was performed at 3 ± 1 days after cardiac arrest. EEG at 24 h and ischemic damage in 21 predefined brain regions on diffusion weighted imaging and fluid-attenuated inversion recovery on a scale from 0 to 4 were related to outcome. Quantitative MRI analyses included mean apparent diffusion coefficient (ADC) and percentage of brain volume with ADC < 450 × 10−6 mm2/s, < 550 × 10−6 mm2/s, and < 650 × 10−6 mm2/s. Poor outcome was defined as a Cerebral Performance Category score of 3–5 at 6 months.
Results
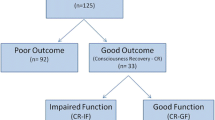
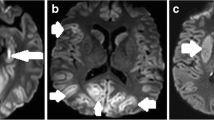
We included 50 patients, of whom 20 (40%) demonstrated poor outcome. Visual EEG assessment correctly identified 3 (15%) with poor outcome and 15 (50%) with good outcome. Visual grading of MRI identified 13 (65%) with poor outcome and 25 (89%) with good outcome. ADC analysis identified 11 (55%) with poor outcome and 3 (11%) with good outcome. EEG and MRI combined could predict poor outcome in 16 (80%) patients at 100% specificity, and good outcome in 24 (80%) at 63% specificity. Ischemic damage was most prominent in the cortical gray matter (75% vs. 7%) and deep gray nuclei (45% vs. 3%) in patients with poor versus good outcome.
Conclusions
Magnetic resonance imaging is complementary with EEG for the prediction of poor and good outcome of patients after cardiac arrest who are comatose at admission.



Similar content being viewed by others
References
Sandroni C, D’Arrigo S, Cacciola S, et al. Prediction of poor neurological outcome in comatose survivors of cardiac arrest: a systematic review. Intensive Care Med. 2020;46(10):1803–51.
Nolan, J.P., Sandroni, C., Bottiger, B.W., et al. European Resuscitation Council and European Society of Intensive Care Medicine guidelines 2021: post-resuscitation care. Intensive Care Med 2021.
Bongiovanni F, Romagnosi F, Barbella G, et al. Standardized EEG analysis to reduce the uncertainty of outcome prognostication after cardiac arrest. Intensive Care Med. 2020;46(5):963–72.
Ruijter BJ, Tjepkema-Cloostermans MC, Tromp SC, et al. Early electroencephalography for outcome prediction of postanoxic coma: A prospective cohort study. Ann Neurol. 2019;86(2):203–14.
Westhall E, Rossetti AO, van Rootselaar A-F, et al. Standardized EEG interpretation accurately predicts prognosis after cardiac arrest. Neurology. 2016;86(16):1482–90.
Hofmeijer J, Beernink TMJ, Bosch FH, et al. Early EEG contributes to multimodal outcome prediction of postanoxic coma. Neurology. 2015;85(2):137–43.
Spalletti M, Carrai R, Scarpino M, et al. Single electroencephalographic patterns as specific and time-dependent indicators of good and poor outcome after cardiac arrest. Clin Neurophysiol. 2016;127(7):2610–7.
Hirsch KG, Fischbein N, Mlynash M, et al. Prognostic value of diffusion-weighted MRI for post-cardiac arrest coma. Neurology. 2020;94(16):e1684–92.
Keijzer HM, Hoedemaekers CWE, Meijer FJA, et al. Brain imaging in comatose survivors of cardiac arrest: Pathophysiological correlates and prognostic properties. Resuscitation. 2018;133:124–36.
Barth R, Zubler F, Weck A, et al. Topography of MR lesions correlates with standardized EEG pattern in early comatose survivors after cardiac arrest. Resuscitation. 2020;149:217–24.
Vanden Berghe S, Cappelle S, De Keyzer F, et al. Qualitative and quantitative analysis of diffusion-weighted brain MR imaging in comatose survivors after cardiac arrest. Neuroradiology. 2020;62(11):1361–9.
Keijzer HM, Hoedemaekers CWE. Timing is everything: Combining EEG and MRI to predict neurological recovery after cardiac arrest. Resuscitation. 2020;149:240–2.
Mlynash M, Campbell DM, Leproust EM, et al. Temporal and spatial profile of brain diffusion-weighted MRI after cardiac arrest. Stroke. 2010;41(8):1665–72.
Bevers MB, Scirica BM, Avery KR, et al. Combination of clinical exam, MRI and EEG to predict outcome following cardiac arrest and targeted temperature management. Neurocrit Care. 2018;29(3):396–403.
Wijdicks EF, Campeau NG, Miller GM. MR imaging in comatose survivors of cardiac resuscitation. AJNR Am J Neuroradiol. 2001;22(8):1561–5.
Bjorklund E, Lindberg E, Rundgren M, et al. Ischaemic brain damage after cardiac arrest and induced hypothermia–a systematic description of selective eosinophilic neuronal death. A neuropathologic study of 23 patients. Resuscitation. 2014;85(4):527–32.
Mettenburg JM, Agarwal V, Baldwin M, Rittenberger JC. Discordant observation of brain injury by MRI and malignant electroencephalography patterns in comatose survivors of cardiac arrest following therapeutic hypothermia. AJNR Am J Neuroradiol. 2016;37(10):1787–93.
Beuchat I, Sivaraju A, Amorim E, et al. MRI-EEG correlation for outcome prediction in postanoxic myoclonus: a multicenter study. Neurology. 2020;95(4):e335–41.
Nolan JP, Soar J, Cariou A, et al. European resuscitation council and european society of intensive care medicine guidelines for post-resuscitation care 2015: section 5 of the European resuscitation council guidelines for resuscitation 2015. Resuscitation. 2015;95:202–22.
Prognose van postanoxisch coma - Neurofysiologisch onderzoek postanoxisch coma. 2019 [cited 2020 30–11–2020]; https://richtlijnendatabase.nl/richtlijn/prognose_van_postanoxisch_coma/neurofysiologisch_onderzoek_postanoxisch_coma.html.
Tjepkema-Cloostermans MC, Hofmeijer J, Hom HW, Bosch FH, vanPutten M. Predicting outcome in postanoxic coma: are ten EEG electrodes enough? J Clin Neurophysiol. 2017;34(3):207–12.
Tjepkema-Cloostermans MC, van Meulen FB, Meinsma G, van Putten MJAM. A Cerebral Recovery Index (CRI) for early prognosis in patients after cardiac arrest. Critical care. 2013;17(5):R252.
Hirsch KG, Mlynash M, Jansen S, et al. Prognostic value of a qualitative brain MRI scoring system after cardiac arrest. J Neuroimaging. 2015;25(3):430–7.
Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62(2):782–90.
Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y. Free water elimination and mapping from diffusion MRI. Magn Resonanc Med. 2009;62(3):717–30.
Soares JM, Marques P, Alves V, Sousa N. A hitchhiker’s guide to diffusion tensor imaging. Front Neurosci. 2013;7:31.
Hirsch KG, Mlynash M, Eyngorn I, et al. Multi-center study of diffusion-weighted imaging in coma after cardiac arrest. Neurocrit Care. 2016;24(1):82–9.
Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–55.
Fischl B, Salat DH, Busa E, et al. Whole brain segmentation. Neuron. 2002;33(3):341–55.
Segonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging. 2007;26(4):518–29.
Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–41.
Velly L, Perlbarg V, Boulier T, et al. Use of brain diffusion tensor imaging for the prediction of long-term neurological outcomes in patients after cardiac arrest: a multicentre, international, prospective, observational, cohort study. Lancet Neurol. 2018;17(4):317–26.
Wouters A, Scheldeman L, Plessers S, et al. Added value of quantitative apparent diffusion coefficient values for neuroprognostication after cardiac arrest. Neurology. 2021;96(21):e2611–8.
Nolan JP, Berg RA, Bernard S, et al. Intensive care medicine research agenda on cardiac arrest. Intensive Care Med. 2017;43(9):1282–93.
van Putten M, Jansen C, Tjepkema-Cloostermans MC, et al. Postmortem histopathology of electroencephalography and evoked potentials in postanoxic coma. Resuscitation. 2019;134:26–32.
Endisch C, Westhall E, Kenda M, et al. Hypoxic-ischemic encephalopathy evaluated by brain autopsy and neuroprognostication after cardiac arrest. JAMA Neurol. 2020;77(11):1430–9.
Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21(10):1133–45.
Medvedeva YV, Ji SG, Yin HZ, Weiss JH. Differential vulnerability of CA1 versus CA3 pyramidal neurons after Ischemia: possible relationship to sources of Zn2+ accumulation and its entry into and prolonged effects on mitochondria. J Neurosci. 2017;37(3):726–37.
Bodranghien F, Bastian A, Casali C, et al. Consensus paper: revisiting the symptoms and signs of cerebellar syndrome. The Cerebellum. 2016;15(3):369–91.
Grech-Sollars M, Hales PW, Miyazaki K, et al. Multi-centre reproducibility of diffusion MRI parameters for clinical sequences in the brain. NMR Biomed. 2015;28(4):468–85.
Wijman CA, Mlynash M, Caulfield AF, et al. Prognostic value of brain diffusion-weighted imaging after cardiac arrest. Ann Neurol. 2009;65(4):394–402.
Acknowledgements
The authors thank Ruud van Kaam, technical physician at the intensive care unit (ICU) of the Radboudumc, Yvonne Teitink, and Helene Vogelensang, research nurses of the ICU at the Rijnstate Hospital, together with the staff of the ICU, radiology, and clinical neurophysiology departments, for constructive assistance in obtaining informed consent and performing electroencephalography measurements and magnetic resonance imaging examinations.
Funding
HMK is funded by the Rijnstate-Radboud promotion fund. CJMK is supported by a clinical established investigator grant of the Dutch Heart Foundation (Grant Number 2012T077) and an ASPASIA grant from The Netherlands Organization for Health Research and Development, ZonMw (Grant Number 015008048). JH is supported by a clinical established investigator grant of the Dutch Heart Foundation (Grant Number 2018T070).
Author information
Authors and Affiliations
Contributions
Conception and design of study and analyses: HMK, MMLHV, FJAM, CWEH, CJMK, and JH. Data collection: HMK, MMLHV, FHB, and CWEH. Data analyses: HMK, MMLHV, FJAM, BART, and JH. Writing of the article (first draft): HMK. Revising of the article: HMK, MMLHV, FJAM, BART, FHB, CWEH, CJMK, and JH. All authors approve of the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors report no conflict of interest.
Ethical approval/informed consent
The study is conducted in accordance with ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The study was approved by the Committee on Research Involving Human Subjects region Arnhem-Nijmegen.
Clinical trial registration
ClinicalTrials.gov: NCT03308305
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Keijzer, H.M., Verhulst, M.M.L.H., Meijer, F.J.A. et al. Prognosis After Cardiac Arrest: The Additional Value of DWI and FLAIR to EEG. Neurocrit Care 37, 302–313 (2022). https://doi.org/10.1007/s12028-022-01498-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-022-01498-z