Abstract
Introduction
Seizures and abnormal periodic or rhythmic patterns are observed on continuous electroencephalography monitoring (cEEG) in up to half of patients hospitalized with moderate to severe traumatic brain injury (TBI). We aimed to determine the impact of seizures and abnormal periodic or rhythmic patterns on cognitive outcome 3 months following moderate to severe TBI.
Methods
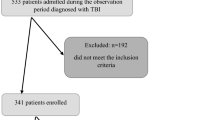
This was a post hoc analysis of the multicenter randomized controlled phase 2 INTREPID2566 clinical trial conducted from 2010 to 2016 across 20 United States Level I trauma centers. Patients with nonpenetrating TBI and postresuscitation Glasgow Coma Scale scores 4–12 were included. Bedside cEEG was initiated per protocol on admission to intensive care, and the burden of ictal-interictal continuum (IIC) patterns, including seizures, was quantified. A summary global cognition score at 3 months following injury was used as the primary outcome.
Results
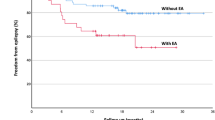
142 patients (age mean + / − standard deviation 32 + / − 13 years; 131 [92%] men) survived with a mean global cognition score of 81 + / − 15; nearly one third were considered to have poor functional outcome. 89 of 142 (63%) patients underwent cEEG, of whom 13 of 89 (15%) had severe IIC patterns. The quantitative burden of IIC patterns correlated inversely with the global cognition score (r = − 0.57; p = 0.04). In multiple variable analysis, the log-transformed burden of severe IIC patterns was independently associated with the global cognition score after controlling for demographics, premorbid estimated intelligence, injury severity, sedatives, and antiepileptic drugs (odds ratio 0.73, 95% confidence interval 0.60–0.88; p = 0.002).
Conclusions
The burden of seizures and abnormal periodic or rhythmic patterns was independently associated with worse cognition at 3 months following TBI. Their impact on longer-term cognitive endpoints and the potential benefits of seizure detection and treatment in this population warrant prospective study.

Modified from study protocols (Neu-2566-TBI-001 and Neu-2566-TBI-002). cEEG, continuous electroencephalography, EFIC, exception from informed consent, ED, emergency department, LAR, legally authorized representative



Similar content being viewed by others
References
Peterson AB, Xu L, Daugherty J, Breiding MJ. Surveillance report of traumatic brain injury-related emergency department visits, hospitalizations, and deaths, United States, 2014.
Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17(1):3–23.
Lee H, Mizrahi MA, Hartings JA, et al. Continuous electroencephalography after moderate to severe traumatic brain injury. Crit Care Med 2019;47(4):574.
Vespa PM, Nuwer MR, Nenov V, et al. Increased incidence and impact of nonconvulsive and convulsive seizures after traumatic brain injury as detected by continuous electroencephalographic monitoring. J Neurosurg. 1999;91(5):750–60.
Vespa PM, Miller C, McArthur D, et al. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit Care Med. 2007;35(12):2830–6.
Vespa P, Tubi M, Claassen J, et al. Metabolic crisis occurs with seizures and periodic discharges after brain trauma. Ann Neurol. 2016;79(4):579–90.
Vespa PM, McArthur DL, Xu Y, et al. Nonconvulsive seizures after traumatic brain injury are associated with hippocampal atrophy. Neurology. 2010;75(9):792–8.
Carney N, Totten AM, O’Reilly C, et al. Guidelines for the Management of Severe Traumatic Brain Injury. Fourth Edition Neurosurgery. 2017;80(1):6–15.
Claassen J, Taccone FS, Horn P, et al. Recommendations on the use of EEG monitoring in critically ill patients: consensus statement from the neurointensive care section of the ESICM. Intensive Care Med. 2013;39(8):1337–51.
Claassen J, Perotte A, Albers D, et al. Nonconvulsive seizures after subarachnoid hemorrhage: Multimodal detection and outcomes. Ann Neurol. 2013;74(1):53–64.
Howell KB, Harvey AS, Archer JS. Epileptic encephalopathy: Use and misuse of a clinically and conceptually important concept. Epilepsia. 2016;57(3):343–7.
Steyerberg EW, Mushkudiani N, Perel P, et al. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med 2008;5(8):e165.
Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma. 1998;15(8):573–85.
Hirsch LJ, LaRoche SM, Gaspard N, et al. American clinical neurophysiology society’s standardized critical care EEG terminology: 2012 version. J Clin Neurophysiol. 2013;30(1):1–27.
Blair JR, Spreen O. Predicting premorbid IQ: A revision of the national adult reading test. Clinical Neuropsychologist. 1989;3(2):129–36.
Johnstone B, Callahan CD, Kapila CJ, Bouman DE. The comparability of the WRAT-R reading test and NAART as estimates of premorbid intelligence in neurologically impaired patients. Arch Clin Neuropsychol. 1996;11(6):513–9.
Watt KJ, O’Carroll RE. Evaluating methods for estimating premorbid intellectual ability in closed head injury. J Neurol Neurosurg Psychiatry. 1999;66(4):474–9.
Mathias JL, Bowden SC, Bigler ED, Rosenfeld JV. Is performance on the Wechsler test of adult reading affected by traumatic brain injury? Br J Clin Psychol. 2007;46(Pt 4):457–66.
Mckay C, Casey J, Wertheimer J, Fichtenberg N. Reliability and validity of the RBANS in a traumatic brain injured sample. Arch Clin Neuropsychol. 2007;22(1):91–8.
Randolph C, Tierney MC, Mohr E, Chase TN. The repeatable battery for the assessment of neuropsychological status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20(3):310–9.
Gelman A. Scaling regression inputs by dividing by two standard deviations. Stat Med. 2008;27(15):2865–73.
Team Rs. RStudio: Integrated Development for R. Boston, MA: RStudio, Inc.; 2015.
Witsch J, Frey H-P, Schmidt JM, et al. Electroencephalographic periodic discharges and frequency-dependent brain tissue hypoxia in acute brain injury. JAMA Neurol. 2017;74(3):301.
Brooks DN. Wechsler Memory Scale performance and its relationship to brain damage after severe closed head injury. J Neurol Neurosurg Psychiatry. 1976;39(6):593–601.
Kinnunen KM, Greenwood R, Powell JH, et al. White matter damage and cognitive impairment after traumatic brain injury. Brain. 2011;134(2):449–63.
Dikmen SS, Machamer JE, Powell JM, Temkin NR. Outcome 3 to 5 years after moderate to severe traumatic brain injury. Arch Phys Med Rehab. 2003;84(10):1449–57.
Holmes GL. Cognitive impairment in epilepsy: the role of network abnormalities. Epileptic Disord. 2015;17(2):101–16.
Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51(4):676–85.
De Marchis GM, Pugin D, Meyers E, et al. Seizure burden in subarachnoid hemorrhage associated with functional and cognitive outcome. Neurology. 2016;86(3):253–60.
Pandharipande PP, Girard TD, Jackson JC, et al. Long-Term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306–16.
Dikmen SS, Machamer JE, Winn HR, Temkin NR. Neuropsychological outcome at 1-year post head injury. Neuropsychology. 1995;9(1):80–90.
Naidech AM, Kreiter KT, Janjua N, et al. Phenytoin Exposure Is Associated With Functional and Cognitive Disability After Subarachnoid Hemorrhage. Stroke. 2005;36(3):583–7.
Naidech AM, Beaumont J, Jahromi B, Prabhakaran S, Kho A, Holl JL. Evolving use of seizure medications after intracerebral hemorrhage: a multicenter study. Neurology. 2017;88(1):52–6.
Naidech AM, Beaumont J, Muldoon K, et al. Prophylactic seizure medication and health-related quality of life after intracerebral hemorrhage. Crit Care Med. 2018;46(9):1480–5.
Szaflarski JP, Sangha KS, Lindsell CJ, Shutter LA. Prospective, randomized, single-blinded comparative trial of intravenous levetiracetam versus phenytoin for seizure prophylaxis. Neurocrit Care. 2010;12(2):165–72.
Khor D, Wu J, Hong Q, et al. Early seizure prophylaxis in traumatic brain injuries revisited: a prospective observational study. World J Surg. 2018;42(6):1727–32.
Nakase-Richardson R, Whyte J, Giacino JT, et al. Longitudinal outcome of patients with disordered consciousness in the nidrr tbi model systems programs. J Neurotrauma. 2012;29(1):59–65.
Funding
The INTREPID2566 trial was supported in part by grants from the U.S. Army Medical Research and Materiel Command (FT) and by Neuren Pharmaceuticals Limited. A preliminary version of this work was completed in partial fulfillment of the Master of Science degree in Clinical and Translational Research in the Division of Epidemiology, University of Cincinnati College of Medicine made possible through an Institutional Clinical and Translational Science Award (NIH/NCATS 1UL1TR001425). Author BF is supported by the National Institute Of Neurological Disorders And Stroke of the National Institutes of Health (K23NS101123). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Defense or the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
BF: conceived and designed the analysis, performed the analysis, wrote the paper, HL: contributed data and/or analysis tools, performed the analysis, critical revisions to the paper, MAM: contributed data and/or analysis tools, JAH: collected the data, critical revisions to the paper, LBN: critical revisions to the paper and relevant content expertise, MP: collected the data, contributed data or analysis tools, FCT: conceived and designed the initial study, collected the data, NZ: performed the analysis, JHK: contributed to performance of the analysis and relevant content expertise. Authorship requirements have been met, and the final manuscript was approved by all authors.
Corresponding author
Ethics declarations
Conflicts of interest
The INTREPID2566 trial was supported in part by grants from the U.S. Army Medical Research and Materiel Command (FT) and by Neuren Pharmaceuticals Limited. B. Foreman received research support through the NIH/NINDS. He also receives research funding through the DOD and NSF and speaking fees from UCH Pharma, Inc. M. Privitera received research funds through Neuren Pharmaceuticals Ltd for directing the Core EEG as part of the INTREPID2566 trial. He also receives grant support from GW/Greenwich and YK, performs EEG interpretation for Sage, Inc. and serves on the DSMB for Astrellas. F.C. Tortella served as principal investigator for the INTREPID2566 trial and received funding through the DOD and Neuren Pharmaceuticals Limited. The remaining authors report no relevant conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Foreman, B., Lee, H., Mizrahi, M.A. et al. Seizures and Cognitive Outcome After Traumatic Brain Injury: A Post Hoc Analysis. Neurocrit Care 36, 130–138 (2022). https://doi.org/10.1007/s12028-021-01267-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-021-01267-4