Abstract
Objective
Severe traumatic brain injury (sTBI) represents a serious public health issue with high morbidity and mortality. Neuroimaging plays a crucial role in the evaluation of sTBI patients. Phosphorous magnetic resonance spectroscopy (31P-MRS) is an imaging technique for evaluation of energy metabolites. The aim of this study is to evaluate the feasibility and the diagnostic potential of ultra-early 31P-MRS to detect changes in cerebral energy metabolism in sTBI.
Methods
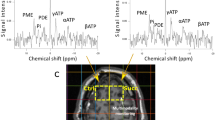
Adult patients with sTBI presenting with GCS ≤ 8 being eligible for MRI were prospectively included in the study and MRI was performed within 72 h after trauma. Imaging was performed using a 3 Tesla MRI. 31P-MRS data from the structurally affected side were compared to data from normal appearing contralateral areas symmetrically to the location of the traumatic lesions, and to data of matched healthy controls.
Results
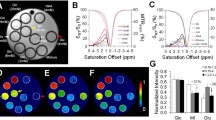
Ten sTBI patients (3 female, 7 male), aged between 20 and 75 years, with a mean initial GCS of 6 were analyzed. MRI was performed 61 h (mean, range 37–71 h) after trauma. Statistical analysis revealed no significant differences between the lesioned side and contralaterally. An increased PCr/ATP ratio and a decreased PME/PDE ratio were present in structurally normal appearing, but traumatized tissue when compared to the healthy population, thus indicating significant differences in ATP resynthesis and membrane turnover (F (2,33), P = 0.005 and, P = 0.027, respectively).
Conclusion
31P-MRS could provide a better understanding of pertinent global changes in cerebral energy metabolism in sTBI patients under general anesthesia.


Similar content being viewed by others
References
Stocchetti N, Le Roux P, Vespa P, et al. Clinical review: neuromonitoring—an update. Crit Care. 2013;17(1):201.
Oddo M, Villa F, Citerio G. Brain multimodality monitoring: an update. Curr Opin Crit Care. 2012;18(2):111–8.
Tavakoli S, Peitz G, Ares W, Hafeez S, Grandhi R. Complications of invasive intracranial pressure monitoring devices in neurocritical care. Neurosurg Focus. 2017;43(5):E6.
Chen R, Ravindra VM, Cohen AL, et al. Molecular features assisting in diagnosis, surgery, and treatment decision making in low-grade gliomas. Neurosurg Focus. 2015;38(3):E2.
Shi XF, Carlson PJ, Sung YH, et al. Decreased brain PME/PDE ratio in bipolar disorder: a preliminary (31) P magnetic resonance spectroscopy study. Bipolar Disord. 2015;17(7):743–52.
Kamble RB, Peruvumba NJ, Shivashankar R. Energy status and metabolism in intracranial space occupying lesions: a prospective 31p spectroscopic study. J Clin Diagn Res. 2014;8(11):RC05–8.
Weber-Fahr W, Englisch S, Esser A, et al. Altered phospholipid metabolism in schizophrenia: a phosphorus 31 nuclear magnetic resonance spectroscopy study. Psychiatry Res. 2013;214(3):365–73.
Van Boven RW, Harrington GS, Hackney DB, et al. Advances in neuroimaging of traumatic brain injury and posttraumatic stress disorder. J Rehabil Res Dev. 2009;46(6):717–57.
Pan JW, Kim JH, Cohen-Gadol A, et al. Regional energetic dysfunction in hippocampal epilepsy. Acta Neurol Scand. 2005;111(4):218–24.
Vink R, Golding EM, Williams JP, McIntosh TK. Blood glucose concentration does not affect outcome in brain trauma: a 31P MRS study. J Cereb Blood Flow Metab. 1997;17(1):50–3.
Vink R, Faden AI, McIntosh TK. Changes in cellular bioenergetic state following graded traumatic brain injury in rats: determination by phosphorus 31 magnetic resonance spectroscopy. J Neurotrauma. 1988;5(4):315–30.
Vink R, McIntosh TK, Weiner MW, Faden AI. Effects of traumatic brain injury on cerebral high-energy phosphates and pH: a 31P magnetic resonance spectroscopy study. J Cereb Blood Flow Metab. 1987;7(5):563–71.
Ishige N, Pitts LH, Berry I, Nishimura MC, James TL. The effects of hypovolemic hypotension on high-energy phosphate metabolism of traumatized brain in rats. J Neurosurg. 1988;68(1):129–36.
Rango M, Lenkinski RE, Alves WM, Gennarelli TA. Brain pH in head injury: an image-guided 31P magnetic resonance spectroscopy study. Ann Neurol. 1990;28(5):661–7.
Fanara B, Manzon C, Barbot O, Desmettre T, Capellier G. Recommendations for the intra-hospital transport of critically ill patients. Crit Care. 2010;14(3):R87.
Stovell, M.G., Mada, M.O., Carpenter, T.A., et al. Phosphorus spectroscopy in acute TBI demonstrates metabolic changes that relate to outcome in the presence of normal structural MRI. J Cereb Blood Flow Metab 2018:271678X18799176.
Garnett MR, Corkill RG, Blamire AM, et al. Altered cellular metabolism following traumatic brain injury: a magnetic resonance spectroscopy study. J Neurotrauma. 2001;18(3):231–40.
Carney N, Totten AM, O’Reilly C, et al. Guidelines for the Management of Severe Traumatic Brain Injury. Fourth Edition. Neurosurgery. 2017;80(1):6–15.
Pinggera, D., Luger, M., Bürgler, I., et al. Safety of early MRI examinations in severe TBI: a test battery for proper patient selection. Front Neurol 2020;11.
Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson. 1997;129(1):35–43.
Liu Y, Gu Y, Yu X. Assessing tissue metabolism by phosphorous-31 magnetic resonance spectroscopy and imaging: a methodology review. Quant Imaging Med Surg. 2017;7(6):707–26.
Xiong KL, Zhu YS, Zhang WG. Diffusion tensor imaging and magnetic resonance spectroscopy in traumatic brain injury: a review of recent literature. Brain Imaging Behav. 2014;8(4):487–96.
Stovell MG, Yan JL, Sleigh A, et al. Assessing metabolism and injury in acute human traumatic brain injury with magnetic resonance spectroscopy: current and future applications. Front Neurol. 2017;8:426.
Maudsley, A.A., Govind, V., Levin, B., et al. Distributions of Magnetic Resonance Diffusion and Spectroscopy Measures with Traumatic Brain Injury. J Neurotrauma 2015.
Poole VN, Abbas K, Shenk TE, et al. MR spectroscopic evidence of brain injury in the non-diagnosed collision sport athlete. Dev Neuropsychol. 2014;39(6):459–73.
Kemp GJ. Non-invasive methods for studying brain energy metabolism: what they show and what it means. Dev Neurosci. 2000;22(5–6):418–28.
Beer M, Seyfarth T, Sandstede J, et al. Absolute concentrations of high-energy phosphate metabolites in normal, hypertrophied, and failing human myocardium measured noninvasively with 31P-SLOOP magnetic resonance spectroscopy. J Am Coll Cardiol. 2002;40(7):1267–74.
Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem J. 1992;281(Pt 1):21–40.
Welch KM, Levine SR, D’Andrea G, Schultz LR, Helpern JA. Preliminary observations on brain energy metabolism in migraine studied by in vivo phosphorus 31 NMR spectroscopy. Neurology. 1989;39(4):538–41.
Albers MJ, Krieger MD, Gonzalez-Gomez I, et al. Proton-decoupled 31P MRS in untreated pediatric brain tumors. Magn Reson Med. 2005;53(1):22–9.
Daly PF, Lyon RC, Faustino PJ, Cohen JS. Phospholipid metabolism in cancer cells monitored by 31P NMR spectroscopy. J Biol Chem. 1987;262(31):14875–8.
Di Pietro V, Amorini AM, Tavazzi B, et al. The molecular mechanisms affecting N-acetylaspartate homeostasis following experimental graded traumatic brain injury. Mol Med. 2014;20:147–57.
Signoretti S, Vagnozzi R, Tavazzi B, Lazzarino G. Biochemical and neurochemical sequelae following mild traumatic brain injury: summary of experimental data and clinical implications. Neurosurg Focus. 2010;29(5):E1.
Smith DH, Cecil KM, Meaney DF, et al. Magnetic resonance spectroscopy of diffuse brain trauma in the pig. J Neurotrauma. 1998;15(9):665–74.
Unterberg AW, Andersen BJ, Clarke GD, Marmarou A. Cerebral energy metabolism following fluid-percussion brain injury in cats. J Neurosurg. 1988;68(4):594–600.
Signoretti S, Di Pietro V, Vagnozzi R, et al. Transient alterations of creatine, creatine phosphate, N-acetylaspartate and high-energy phosphates after mild traumatic brain injury in the rat. Mol Cell Biochem. 2010;333(1–2):269–77.
Bolton AN, Saatman KE. Regional neurodegeneration and gliosis are amplified by mild traumatic brain injury repeated at 24-hour intervals. J Neuropathol Exp Neurol. 2014;73(10):933–47.
Fehily B, Fitzgerald M. Repeated mild traumatic brain injury: potential mechanisms of damage. Cell Transplant. 2017;26(7):1131–55.
Steen C, Wilczak N, Hoogduin JM, Koch M De, Keyser J. Reduced creatine kinase B activity in multiple sclerosis normal appearing white matter. PLoS ONE. 2010;5(5):e10811.
Langan C, McDonald C. Neurobiological trait abnormalities in bipolar disorder. Mol Psychiatry. 2009;14(9):833–46.
Zaninotto AL, Vicentini JE, Fregni F, et al. Updates and current perspectives of psychiatric assessments after traumatic brain injury: a systematic review. Front Psychiatry. 2016;7:95.
Perry DC, Sturm VE, Peterson MJ, et al. Association of traumatic brain injury with subsequent neurological and psychiatric disease: a meta-analysis. J Neurosurg. 2016;124(2):511–26.
Zgaljardic DJ, Seale GS, Schaefer LA, et al. Psychiatric disease and post-acute traumatic brain injury. J Neurotrauma. 2015;32(23):1911–25.
Nilsson L, Siesjo BK. Influence of anaesthetics on the balance between production and utilization of energy in the brain. J Neurochem. 1974;23(1):29–36.
McKee AC, Daneshvar DH. The neuropathology of traumatic brain injury. Handb Clin Neurol. 2015;127:45–66.
Belanger HG, Vanderploeg RD, Curtiss G, Warden DL. Recent neuroimaging techniques in mild traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2007;19(1):5–20.
Acknowledgments
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
All authors have declared that they have no conflict of interest.
Ethical Approval
The study was approved by the local ethics committee of the Medical University Innsbruck (Protocol number: AN2014-0201 339/4.6). Written informed consent was obtained according to Austrian law either from the patient when regaining consciousness and legal capacity, or from her/his guardian.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pinggera, D., Steiger, R., Bauer, M. et al. Cerebral Energy Status and Altered Metabolism in Early Severe TBI: First Results of a Prospective 31P-MRS Feasibility Study. Neurocrit Care 34, 432–440 (2021). https://doi.org/10.1007/s12028-020-01042-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-020-01042-x