Abstract
The metabolic response to injury is well described; however, very little is understood about optimal markers to measure this response. This summary will address the current evidence about monitoring nutritional status including blood glucose after acute brain injury (ABI). An electronic literature search was conducted for English language articles describing the testing, utility, and optimal methods to measure nutritional status and blood glucose levels in the neurocritical care population. A total of 45 articles were included in this review. Providing adequate and timely nutritional support can help improve outcome after ABI. However, the optimal content and total nutrition requirements remain unclear. In addition, how best to monitor the nutritional status in ABI is still being elucidated, and at present, there is no validated optimal method to monitor the global response to nutritional support on a day-to-day basis in ABI patients. Nitrogen balance may be monitored to assess the adequacy of caloric intake as it relates to protein energy metabolism, but indirect calorimetry, anthropometric measurement, or serum biomarker requires further validation. The adverse effects of hyperglycemia in ABI are well described, and data indicate that blood glucose should be carefully controlled in critically ill patients. However, the optimal frequency or duration for blood glucose monitoring after ABI remains poorly defined. There are significant knowledge gaps about monitoring nutritional status and response to nutritional interventions in ABI; these need to be addressed and hence few recommendations can be made. The optimal frequency and duration of blood glucose monitoring need further study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurological injuries induce a cascade of sympathetic nervous system activation and inflammatory response resulting in a well-described hypermetabolic response [1]. The hypermetabolic response is characterized by protein catabolism and altered gluconeogenesis, weight loss, and disruption of mitochrondrial function in the brain and systemic tissues. Subsequently, increased energy expenditure can have significant impact on substrate utilization and lead to a malnourished state. The primary rational for nutritional therapy is to prevent malnutrition, maintain glucose homeostasis, and prevent the myriad associated complications by providing the appropriate doses of nutrients to meet the calculated or measured needs. Unfortunately, there is sparse data available on optimal methods to monitor nutritional requirements in the neurocritical care setting, limiting the ability to understand the adequacy of nutritional support and minimize the impact of malnourishment.
The adverse effects of hyperglycemia in neurocritical care patients are well described [2]. Altered glucose metabolism is one of the most common clinically observed metabolic abnormalities in the critically ill patient. Serum glucose is frequently elevated and the response to a glucose load demonstrates the problem of insulin resistance commonly seen during acute illness. Elevations in serum glucose levels appear to result primarily from sympathetic activation, inflammation and resultant increased hepatic glucose production rather than decreased glucose utilization. The rate of glucose oxidation in the periphery increases after injury when a stable circulation is maintained; however, the hepatic glucose output increases dramatically. Under the influence of increased concentration of catecholamines, glucagon and cortisol, glucose is synthetized in the liver using lactic acid, pyruvic acid, and amino acid as substrates [3]. Data indicate that blood glucose should be carefully controlled in critically ill patients; however, to what level and how need further clarification [2]. Central to this management is a strategy to accurately assess glucose levels to understand the response to injury and intensive insulin therapy.
Methods
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [4].
Search
Using the PubMed database, a systematic review was performed (1966–August 2013) using the flowing MESH terms: nutrition, nutritional support, nitrogen balance, catabolism, energy expenditure, hypermetabolism, and glucose AND monitoring. We restricted our search to articles in the English and describing critically ill patients with at least one of the following: traumatic brain injury (TBI), SAH, intracranial hemorrhage, ischemic stroke, coma after cardiac arrest, seizures.
Study Selection and Data Collection
The authors independently reviewed citations, abstracts and full-text articles to select eligible studies. Unpublished data or congress presentations/abstracts were not considered. We excluded: a) review articles; b) case reports or case series with ≤5 patients; c) experimental studies; d) study on pediatric ICU populations (<18years); e) studies that were not conducted on ICU patients; f) studies dealing with patients in brain death. Data were abstracted using a predefined abstraction spreadsheet, according to the PICO system.
Review end-points
The end-points of this review were to answer the following questions about monitoring of nutritional status and systemic glucose levels in acute brain injuries (ABI).
Nutritional Status
-
1.
Can measuring energy expenditure with indirect calorimetry be used to monitor the nutritional requirements in neurocritical care patients?
-
2.
What methods are useful when monitoring the response to nutritional interventions?
-
3.
Is there utility in monitoring gastric residuals in patients receiving enteral nutrition?
Blood Glucose
-
1.
Should monitoring of serial blood glucose values be performed routinely during the critical care after acute brain injury?
-
2.
How should glucose monitoring be performed in the acute critical care period after brain injury?
-
3.
What duration of frequent glucose monitoring is required after brain injury?
-
4.
Is there an optimal point of care method to monitor blood glucose levels?
Literature Summary
The search identified 7,747 studies; 5,549 of these were selected because they were studies completed in humans. From this list, 91 articles were found to address the questions proposed in the PICO approach. Ultimately, 45 published articles were used in the analyses. After selection, the evidence was classified and practical recommendations developed according to the GRADE system.
Nutritional Status and Assessment
Although it is recognized that providing adequate, timely nutritional support improves outcomes in neurocritical care populations [5], both optimal content and total nutrition requirements remain unclear. Very few data are available about the appropriate parameters to monitor nutritional status. The majority of published studies investigate nutritional status after traumatic brain injuries (TBI). Much of this literature also is more pertinent to the rehabilitation phase of injury and not the critical care setting. Consequently, many commonly used strategies to monitor nutrition progress, such as body weight and other anthropormetric measures are not easily applicable in the intensive care unit (ICU) setting since there is concomitant use of sedatives or therapeutics, inflammatory responses and sympathetic activation that directly impact the metabolic rate.
Can Measuring Energy Expenditure with Indirect Calorimetry be used to Monitor the Nutritional Requirements in Neurocritical care Patients?
We were not able to identify any studies that answered this question in our review. Indirect calorimetry (IDC) assesses the CO2 production and O2 consumption directly from the patient and using the modified Weir equation, computes the resting energy expenditure (REE) [6]. IDC is the gold standard by which to measure the REE in critically ill patients. Several studies, most in TBI, have used IDC values to determine the REE after brain injury [7]. In four TBI studies, singular REE measurements were used to determine the amount of calories required for experimental nutritional interventions [8–11]. These studies failed to identify an optimal timing, duration, or frequency for REE measurements. In 3 studies in non-TBI brain hemorrhage patients, REE was measured as a marker of hypermetabolism and not to guide nutritional therapy [12–14]. No study has assessed the accuracy of repeated measures of REE during the acute injury to monitor the ongoing nutritional requirements in the neurocritical care patient population.
We identified 4 studies that examined whether any estimating equations adequately assess nutritional requirements acutely after neurological injury? Predictive equations, such as the Harris-Benedict equation, have long been used to determine a patient’s predicted energy expenditure to set a caloric target. So-called activity and stress factors are used to improve the equation’s accuracy and numerous estimating equations have been developed for specific critical care populations [15]. Predictive equations have been validated to REE but not necessarily to actual nutritional requirements. There are four studies in TBI and one in ischemic stroke that utilized specific predictive equations to test accuracy to REE measurements [16–20]; however none of these studies determined the accuracy of these equations to define nutritional requirements or monitor ongoing level of nutritional support. No studies in other neurocritical care patient populations were identified.
What Methods are Useful when Monitoring the Response to Nutritional Interventions?
Thirteen studies that addressed this question were reviewed. Protein energy metabolism is commonly assessed by measurement of nitrogen balance derived from the difference between daily nitrogen intake and daily nitrogen output. Nitrogen intake is easily calculated from the volume and the composition of all the fluids administered to the patient. Nitrogen loss is measured by collecting urine, feces, and drainage fluids and by determining their nitrogen, or alternatively by only measuring urine urea nitrogen values and using an adjustment factor for nitrogen losses elsewhere. The nitrogen balance is the only biomarker for protein energy metabolism widely reported in the neurocritical care population [8, 9, 16, 18, 21]. RCTs measuring nitrogen balance or the degree of nitrogen loss as a surrogate of outcome have been performed and suggest that less than 50 % of administered nitrogen is retained after TBI [22–24]. In ischemic stroke and non-traumatic brain hemorrhage, nitrogen balance has been shown to be associated with severity of injury and infectious complications [13, 25] but not as a marker of appropriate nutritional therapy. The majority of studies reported nitrogen balance once during the first week of injury. The acceptable amount of nitrogen loss relating it to functional recovery has not been subjected to clinical studies.
There is no validated way to monitor the global response to nutritional support on a day-to-day basis. A short-term biochemical parameter that could reliably monitor response to nutrition within the same timeframe as the nutrition therapy would be very valuable in clinical practice. Both serum albumin and transthyretin levels are widely used markers of nutritional therapy in critical illness [26]. There are no studies investigating the utility of monitoring either albumin or transthyretin as markers of responsiveness to nutritional therapy in the neurocritical care patient population. Body weight is the most commonly used indicator in assessing nutrition status in non-critical care population [26], but in the ICU setting is more likely a marker of fluid balance than of nutritional status and not a reliable measure of responsiveness to nutritional therapy.
Is there utility in monitoring gastric residuals in patients receiving enteral nutrition?
Early enteral nutrition is the standard of care for all mechanically ventilated critically ill patients. However, enteral nutrition often is not adequately delivered because of concern for aspiration and ventilator associated pneumonia (VAP) related to gastrointestinal intolerance, gastroesophageal reflux and regurgitation or vomiting. We identified one study, a prospective RCT conducted in a mixed critical care population of which 15 % were admitted with primary neurological illnesses that examined the question above. The No reduction in VAP in mechanically ventilated patients who had gastric residuals routinely monitored was observed [27]. In addition patients who did not undergo routine monitoring of gastric residuals were more likely to receive 100 % of their caloric goal. We did not identify any studies that analyzed the utility of monitoring gastric residuals in non-ventilated ABI patients.
Blood Glucose
Recent studies of glycemic control with intensive insulin therapy (IIT) in critical care have resulted in consensus guidelines that recommend relatively higher targets for blood-glucose levels than initially indicated by the original randomized clinical trial (RCT) published in 2001 [28]. Utilizing appropriate modalities to monitor blood glucose levels during IIT is crucial. However, there are very few studies to guide clinicians as to the optimal method to monitor blood glucose levels. Central laboratory blood glucose measurements in plasma are considered the standard since these measures best approximate the physiologic activity of glucose [29]. However, because of convenience and rapidity of results, point-of-care (POC) tests are commonly used to measure blood-glucose concentrations in critically ill patients. Many of these monitors were not designed to monitor patients in the ICU, and as a result may not be a good measure of the physiologic burden of glycemic levels after injury nor sufficiently accurate to guide IIT [30].
Should Monitoring of Serial Blood Glucose Values be Performed Routinely During the Critical care After Acute Brain Injury?
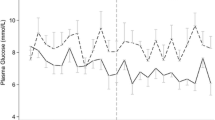
Acute hyperglycemia is common after TBI and other neurologic disorders. Serum glucose concentrations often are increased at admission to hospital and for many days of intensive care. The increase in serum glucose is thought to result from a generalized increase in sympathetic catecholamine tone. The extent of hyperglycemia at admission is correlated with the severity of injury [31, 32] and with poor outcome after TBI [31, 33, 34], with glucose values >300 mg/dl (16.6 mmol/L) uniformly associated with fatal TBI in one study [34]. In particular admission hypergblycemia is associated with poor prognosis [34, 35]. However the relationship of of delayed hyperglycemia >200 mg/dL (11.1 mmol/L) with poor outcome is less certain [32]. Experimentally, immediate but not persistent post-traumatic hyperglycemia may exacerbate final lesion volume, but this is not well studied in humans. Furthermore it is unclear whether one-time or persistent hyperglycemia has a greater negative impact on brain injury. The association between hyperglycemia and poor outcome also is observed in ischemic stroke, intracerebral hemorrhage and subarachnoid hemorrhage [36–38]. Despite these repeated associations, it is not clear whether hyperglycemia is an independent factor or a marker of disease severity.
Hyperglycemia is thought to worsen outcome after ABI through downstream metabolic effects. It is generally thought that hyperglycemia will result in increased anaerobic metabolism and lactate production under conditions of brain ischemia. Post-traumatic brain ischemia, which is thought to be a common phenomenon in the hours after TBI, potentially could be made worse by hyperglycemia. Two recent reports support this theory. In the first, Diaz-Parejo et al. [39] demonstrated increased extracellular lactate during episodes of profound hyperglycemia, with serum glucose >15 mmol/L (270 mg/dL), but not with moderate hyperglycemia in which serum glucose was 12–15 mmol/L (216–270 mg/dL). In the second study, Zygun et al. [40] confirmed that profound hyperglycemia is associated with worsened tissue acidosis in TBI. In addition, hyperglycemia is associated with other adverse effects in critically ill patients, such as increased infection rates, poor wound healing, and increased mortality. Thus, the preponderance of evidence suggests that hyperglycemia has deleterious effects after ABI and hence there is value to its assessment and monitoring.
However there are no studies that specifically address with what frequency or for how long should blood glucose be monitored after brain injury. The majority of studies of the impact of serum glucose on outcome have monitored serum glucose or blood capillary levels at a frequency of every 1–8 h [31]. The frequency of monitoring is driven by IIT protocols. In prospective and retrospective studies of IIT, the usual frequency is every 1–2 h. There is no evidence to suggest a specific frequency for monitoring that is a more reliable marker of the physiologic impact of blood glucose levels after brain injury. Beyond admission, the majority of studies have indicated that glucose levels for the next 48 h and through the period of critical care remain important predictors of long term outcome [31]. However, the duration of critical care is different across studies, and therefore optimal duration cannot be determined.
How Should Glucose Monitoring be Performed in the Acute Critical care Period After Brain Injury?
Common methods used to measure blood glucose include arterial measurements from arterial blood gas analyzers (ABGs) or glucose meters that use either arterial or capillary blood. There are no studies specifically performed in neurocritical care populations; however, we identified 13 studies prospective and retrospective studies in general critical care populations that examine point of care testing to set glycemic targets. In summary these studies demonstrate blood-glucose monitoring with ABG analyzers tend to be more accurate than that by glucose meters with arterial blood. Additional studies have noted greater overall accuracy with arterial blood samples versus capillary blood samples for blood-glucose measurements in adult critically ill patient [41–53]. It is important to note that the accuracy of blood-glucose measurements is variable regardless of the type of device, indicating that all POC devices require routine calibration with central laboratory glucose measurements to ensure continued accuracy. Finally, seven studies noted that POC testing of any kind is less accurate during episodes of hypoglycemia or hemodynamic shock [42, 45–50].
Limitations to the Literature
Although studies have outlined the importance of early, adequate nutritional support and blood glucose monitoring after brain injury [5, 44], there is a paucity of studies assessing the natural history of the metabolic response to acute neurological injury. Developing metabolic profiles of different types of neurological injury will better inform the overall approach towards overall nutritional support and targeted blood glucose monitoring. This will necessitate the use of newer analytical techniques that allow for better description of the response to injury at the cellular level. Any new nutritional biomarker that is developed will need to be validated in a clinical setting where there is a significant interaction between inflammation and the sympathetic activation and the metabolic response. This area of monitoring also is ideally suitable to understand the effects of substrates and substrate utilization on gene expression, and should represent an area for future investigation. Finally, there is a need for indicators of nutrition progress that can be monitored sequentially, where the change in the indicator over time is predictive of an improved nutritional therapy related outcome. The most commonly utilized methods, indirect calorimetry and nitrogen balance, have predominantly been studied as singular assessments of physiological state, rather than as markers of responsiveness to nutritional therapies. This may be due to the inability of these markers to change significantly within the timeframe of a short-term response to nutrition therapy. This lack of sensitivity renders current monitoring techniques useless when assessing whether the nutrition provided is optimal.
With glucose monitoring, further work is needed to improve the accuracy of POC tests and to better define the duration of close monitoring that coincide with the greatest risk for secondary injury. An area of interest is determining the relationship between blood and interstitial brain glucose levels. Future investigations in nutritional and blood glucose monitoring should evaluate the impact the systemic inflammatory response and/or therapies may directly impact metabolism, such as fever, targeted temperature modulation or sedation, into the assessment of the nutritional requirements of ABI patients.
References
Clifton GL, Robertson CS, Grossman RG, Hodge S, Foltz R, Garza C. The metabolic response to severe head injury. J Neurosurg. 1984;60:687–96.
Godoy DA, Di Napoli M, Rabinstein AA. Treating hyperglycemia in neurocritical patients: benefits and perils. Neurocrit Care. 2010;13:425–38.
Oddo M, Schmidt JM, Mayer SA, Chiolero RL. Glucose control after severe brain injury. Curr Opin Clin Nutr Metab care. 2008;11:134–9.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–12.
Dennis MS, Lewis SC, Warlow C, Collaboration FT. Routine oral nutritional supplementation for stroke patients in hospital (FOOD): a multicentre randomised controlled trial. Lancet. 2005;365:755–63.
Guttormsen AB, Pichard C. Determining energy requirements in the ICU. Curr Opin Clin Nutr Metab care. 2014;17:171–6.
Krakau K, Omne-Ponten M, Karlsson T, Borg J. Metabolism and nutrition in patients with moderate and severe traumatic brain injury: a systematic review. Brain Inj. 2006;20:345–67.
Borzotta AP, Pennings J, Papasadero B, et al. Enteral versus parenteral nutrition after severe closed head injury. J Trauma. 1994;37:459–68.
Hatton J, Rapp RP, Kudsk KA, et al. Intravenous insulin-like growth factor-I (IGF-I) in moderate-to-severe head injury: a Phase II safety and efficacy trial. Neurosurg Focus. 1997;2:ECP1 discussion p following ECP.
Kudsk KA, Mowatt-Larssen C, Bukar J, et al. Effect of recombinant human insulin-like growth factor I and early total parenteral nutrition on immune depression following severe head injury. Arch Surg (Chicago, Ill : 1960) 1994;129:66–70; discussion-1.
Young B, Ott L, Kasarskis E, et al. Zinc supplementation is associated with improved neurologic recovery rate and visceral protein levels of patients with severe closed head injury. J Neurotrauma. 1996;13:25–34.
Esper DH, Coplin WM, Carhuapoma JR. Energy expenditure in patients with nontraumatic intracranial hemorrhage. J Parenter Enter Nutr. 2006;30:71–5.
Kasuya H, Kawashima A, Namiki K, Shimizu T, Takakura K. Metabolic profiles of patients with subarachnoid hemorrhage treated by early surgery. Neurosurgery. 1998;42:1268–74 discussion 74–5.
Badjatia N, Carpenter A, Fernandez L, et al. Relationship between C-reactive protein, systemic oxygen consumption, and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Stroke. 2011;42:2436–42.
Schlein KM, Coulter SP. Best practices for determining resting energy expenditure in critically ill adults. Nutr Clin Pract. 2014;29:44–55.
Taylor SJ, Fettes SB, Jewkes C, Nelson RJ. Prospective, randomized, controlled trial to determine the effect of early enhanced enteral nutrition on clinical outcome in mechanically ventilated patients suffering head injury. Crit Care Med. 1999;25:25–31.
Sacks GS, Brown RO, Teague D, Dickerson RN, Tolley EA, Kudsk KA. Early nutrition support modifies immune function in patients sustaining severe head injury. JPEN. 1995;19:387–92.
Ritter AM, Robertson CS, Goodman JC, Contant CF, Grossman RG. Evaluation of a carbohydrate-free diet for patients with severe head injury. J Neurotrauma. 1996;13:473–85.
Minard G, Kudsk KA, Melton S, Patton JH, Tolley EA. Early versus delayed feeding with an immune-enhancing diet in patients with severe head injuries. JPEN. 2000;24:145–9.
Frankenfield DC, Ashcraft CM. Description and prediction of resting metabolic rate after stroke and traumatic brain injury. Nutrition (Burbank, Los Angeles County, Calif). 2012;28:906–11.
Weekes E, Elia M. Observations on the patterns of 24-hour energy expenditure changes in body composition and gastric emptying in head-injured patients receiving nasogastric tube feeding. JPEN. 1996;20:31–7.
Clifton GL, Robertson CS, Contant CF. Enteral hyperalimentation in head injury. J Neurosurg. 1985;62:186–93.
Hausmann D, Mosebach KO, Caspari R, Rommelsheim K. Combined enteral-parenteral nutrition versus total parenteral nutrition in brain-injured patients. A comparative study. Intensive Care Med. 1985;11:80–4.
Dominioni L, Trocki O, Fang CH, et al. Enteral feeding in burn hypermetabolism: nutritional and metabolic effects of different levels of calorie and protein intake. JPEN. 1985;9:269–79.
Chalela JA, Haymore J, Schellinger PD, Kang DW, Warach S. Acute stroke patients are being underfed: a nitrogen balance study. Neurocrit Care. 2004;1:331–4.
Ferrie S, Allman-Farinelli M. Commonly used “nutrition” indicators do not predict outcome in the critically ill: a systematic review. Nutrit Clin Pract. 2013;28:463–84.
Reignier J, Mercier E, Le Gouge A, et al. Effect of not monitoring residual gastric volume on risk of ventilator-associated pneumonia in adults receiving mechanical ventilation and early enteral feeding: a randomized controlled trial. JAMA. 2013;309:249–56.
Jacobi J, Bircher N, Krinsley J, et al. Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit Care Med. 2012;40:3251–76.
D’Orazio P, Burnett RW, Fogh-Andersen N, et al. Approved IFCC recommendation on reporting results for blood glucose (abbreviated). Clin Chem. 2005;51:1573–6.
Scott MG, Bruns DE, Boyd JC, Sacks DB. Tight glucose control in the intensive care unit: are glucose meters up to the task? Clin Chem. 2009;55:18–20.
Lam AM, Winn HR, Cullen BF, Sundling N. Hyperglycemia and neurological outcome in patients with head injury. J Neurosurg. 1991;754:54551.
Margulies DR, Hiatt JR, Vinson D Jr, Shabot MM. Relationship of hyperglycemia and severity of illness to neurologic outcome in head injury patients. Am Surg. 1994;60(6):38790.
Rovlias A, Kotsou S. The influence of hyperglycemia on neurological outcome in patients with severe head injury. Neurosurgery. 2000;46(2):33542.
Cochran A, Scaife ER, Hansen KW, Downey EC. Hyperglycemia and outcomes from pediatric traumatic brain injury. J Trauma. 2003;55:10358.
Kramer AH, Roberts DJ, Zygun DA. Optimal glycemic control in neurocritical care patients: a systematic review and meta-analysis. Crit Care (London, England). 2012;16:R203.
Lanzino G, Kassell NF, Germanson T, Truskowski L, Alves W. Plasma glucose levels and outcome after aneurysmal subarachnoid hemorrhage. J Neurosurg. 1993;79(6):88591.
Bruno A, Levine SR, Frankel MR, Brott TG, Lin Y, Tilley BC, Lyden PD, Broderick JP, Kwiatkowski TG, Fineberg SE, NINDS rtPA Stroke Study Group. Admission glucose level and clinical outcomes in the NINDS rtPA Stroke Trial. Neurology. 2002;59:66974.
Passero S, Ciacci G, Ulivelli M. The influence of diabetes and hyperglycemia on clinical course after intracerebral hemorrhage. Neurology. 2003;61:13516.
Diaz-Parejo P, Stahl N, Xu W, Reinstrup P, Ungerstedt U, Nordstrom CH. Cerebral energy metabolism during transient hyperglycemia in patients with severe brain trauma. Intensive Care Med. 2003;29(4):54450.
Zygun DA, Steiner LA, Johnston AJ, Hutchinson PJ, AlRawi PG, Chatfield D, Kirkpatrick PJ, Menon DK, Gupta AK. Hyperglycemia and brain tissue pH after traumatic brain injury. Neurosurgery. 2004;55(4):877–81.
Stadlbauer V, Wallner S, Stojakovic T, Smolle KH. Comparison of 3 different multianalyte point-of-care devices during clinical routine on a medical intensive care unit. J Crit Care. 2011;26(433):e1–11.
Corstjens AM, Ligtenberg JJ, van der Horst IC, et al. Accuracy and feasibility of point-of-care and continuous blood glucose analysis in critically ill ICU patients. Crit care (London, England). 2006;10:R135.
Hoedemaekers CW, Klein Gunnewiek JM, Prinsen MA, Willems JL, Van der Hoeven JG. Accuracy of bedside glucose measurement from three glucometers in critically ill patients. Crit Care Med. 2008;36:3062–6.
Slater-MacLean L, Cembrowski G, Chin D, et al. Accuracy of glycemic measurements in the critically ill. Diabetes Technol Ther. 2008;10:169–77.
Kanji S, Buffie J, Hutton B, et al. Reliability of point-of-care testing for glucose measurement in critically ill adults. Crit Care Med. 2005;33:2778–85.
Petersen JR, Graves DF, Tacker DH, Okorodudu AO, Mohammad AA, Cardenas VJ Jr. Comparison of POCT and central laboratory blood glucose results using arterial, capillary, and venous samples from MICU patients on a tight glycemic protocol. Clini Chim Acta. 2008;396:10–3.
Desachy A, Vuagnat AC, Ghazali AD, et al. Accuracy of bedside glucometry in critically ill patients: influence of clinical characteristics and perfusion index. Mayo Clin proc Mayo Clin. 2008;83:400–5.
Lonjaret L, Claverie V, Berard E, et al. Relative accuracy of arterial and capillary glucose meter measurements in critically ill patients. Diabetes Metab. 2012;38:230–5.
Critchell CD, Savarese V, Callahan A, Aboud C, Jabbour S, Marik P. Accuracy of bedside capillary blood glucose measurements in critically ill patients. Intensive Care Med. 2007;33:2079–84.
Meynaar IA, van Spreuwel M, Tangkau PL, et al. Accuracy of AccuChek glucose measurement in intensive care patients. Crit Care Med. 2009;37:2691–6.
Cook A, Laughlin D, Moore M, et al. Differences in glucose values obtained from point-of-care glucose meters and laboratory analysis in critically ill patients. Am J Crit care. 2009;18:65–71 quiz 2.
Karon BS, Gandhi GY, Nuttall GA, et al. Accuracy of roche accu-chek inform whole blood capillary, arterial, and venous glucose values in patients receiving intensive intravenous insulin therapy after cardiac surgery. Am J Clin Pathol. 2007;127:919–26.
Finkielman JD, Oyen LJ, Afessa B. Agreement between bedside blood and plasma glucose measurement in the ICU setting. Chest. 2005;127:1749–51.
Acknowledgments
Neeraj Badjatia receives Grant Funding from NIH, DOD; is a consultant for Bard and Medivance and is a Scientific Advisor to Cumberland Pharmaceuticals. Paul Vespa receives Grant Funding from NIH, DOD; is a consultant for Edge Therapeutics; and has Stock Options with Intouch Health.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
The Participants of the International Multi-disciplinary Consensus Conference on Multimodality Monitoring are listed in the Appendix.
Appendix: Participants in the International Multi-disciplinary Consensus Conference on Multimodality Monitoring
Appendix: Participants in the International Multi-disciplinary Consensus Conference on Multimodality Monitoring
Peter Le Roux, MD, FACS
Brain and Spine Center
Suite 370, Medical Science Building
Lankenau Medical Center
100 East Lancaster Avenue, Wynnewood, PA 19096, USA
Tel: +1 610 642 3005
Fax: 610 642 3057
lerouxp@mlhs.org
David K Menon MD PhD FRCP FRCA FFICM FMedSci
Head, Division of Anaesthesia, University of Cambridge
Consultant, Neurosciences Critical Care Unit
Box 93, Addenbrooke’s Hospital
Cambridge CB2 2QQ, UK
dkm13@wbic.cam.ac.uk
Paul Vespa, MD, FCCM, FAAN, FNCS
Professor of Neurology and Neurosurgery
Director of Neurocritical Care
David Geffen School of Medicine at UCLA
Los Angeles, CA 90095 USA
PVespa@mednet.ucla.edu
Giuseppe Citerio
Director NeuroIntensive Care Unit
Department of Anesthesia and Critical Care
Ospedale San Gerardo, Monza
Via Pergolesi 33, Monza 20900, Italy
g.citerio@hsgerardo.org
Mary Kay Bader RN, MSN, CCNS, FAHA, FNCS
Neuro/Critical Care CNS
Mission Hospital
Mission Viejo CA 92691, USA
Marykay.Bader@stjoe.org
Gretchen M. Brophy, PharmD, BCPS, FCCP, FCCM
Professor of Pharmacotherapy & Outcomes Science and Neurosurgery
Virginia Commonwealth University
Medical College of Virginia Campus
410 N. 12th Street
Richmond, Virginia 23298-0533 USA
gbrophy@vcu.edu
Michael N. Diringer, MD
Professor of Neurology, Neurosurgery & Anesthesiology
Chief, Neurocritical Care Section
Washington University
Dept. of Neurology, Campus Box 8111
660 S Euclid Ave
St Louis, MO 63110 USA
diringerm@neuro.wustl.edu
Nino Stocchetti, MD
Professor of Anesthesia and Intensive Care
Department of physiopathology and transplant
Milan University
Director Neuro ICU
Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico
Via F Sforza, 35 20122 Milan Italy
e-mail stocchet@policlinico.mi.it
Walter Videtta, MD
ICU Neurocritical Care
Hospital Nacional ‘Prof. a. Posadas’
El Palomar - Pcia. de Buenos Aires
Argentina
wvidetta@ar.inter.net
Rocco Armonda, MD
Department of Neurosurgery
MedStar Georgetown University Hospital
Medstar Health, 3800 Reservoir Road NW
Washington DC 20007
USA
Rocco.Armonda@gmail.com
Neeraj Badjatia, MD, MS, FCCM
Associate Professor of Neurology
University of Maryland School of Medicine Shock Trauma Neurocritical Care
22 S. Greene Street, G7K19
Baltimore, MD 21201
USA
nbadjatia@som.umaryland.edu
Julian Boesel, MD
Department of Neurology
Ruprect-Karls University
Hospital Heidelberg, Im Neuenheimer Feld 400
D-69120 Heidelberg
Germany
Julian.Boesel@med.uni-heidelberg.de
Randal Chesnut, MD, FCCM, FACS
Harborview Medical Center
University of Washington Mailstop 359766
325 Ninth Ave
Seattle WA 98104-2499
USA
chesnutr@u.washington.edu
Sherry Chou, MD, MMSc
Department of Neurology
Brigham and Women’s Hospital
75 Francis Street
Boston MA 02115
USA
schou1@partners.org
Jan Claassen, MD, PhD, FNCS
Assistant Professor of Neurology and Neurosurgery
Head of Neurocritical Care and Medical Director of the Neurological Intensive Care Unit
Columbia University College of Physicians & Surgeons
177 Fort Washington Avenue, Milstein 8 Center room 300
New York, NY 10032
USA
jc1439@cumc.columbia.edu
Marek Czosnyka, PhD
Department of Neurosurgery
University of Cambridge
Addenbrooke’s Hospital, Box 167
Cambridge, CB20QQ
United Kingdom
mc141@medschl.cam.ac.uk
Michael De Georgia, MD
Professor of Neurology
Director, Neurocritical Care Center
Co-Director, Cerebrovascular Center
University Hospital Case Medical Center
Case Western Reserve University School of Medicine
11100 Euclid Avenue
Cleveland, Ohio 44106
michael.degeorgia@uhhospitals.org
Anthony Figaji, MD, PhD
Head of Pediatric Neurosurgery
University of Cape Town
617 Institute for Child Health
Red Cross Children’s Hospital
Rondebosch, 7700 Cape Town
South Africa
anthony.figaji@uct.ac.za
Jennifer Fugate, DO
Department of Neurology
Mayo Clinic
200 First Street SW
Rochester, MN 55905
Fugate.Jennifer@mayo.edu
Raimund Helbok, MD
Department of Neurology, Neurocritical Care Unit
Innsbruck Medical University
Anichstr.35, 6020
Innsbruck,
Austria
raimund.helbok@uki.at
David Horowitz, MD
Associate Chief Medical Officer
University of Pennsylvania Health System
3701 Market Street
Philadelphia, PA, 19104
USA
david.horowitz@uphs.upenn.edu
Peter Hutchinson, MD
Professor of Neurosurgery
NIHR Research Professor
Department of Clinical Neurosciences
University of Cambridge
Box 167 Addenbrooke’s Hospital
Cambridge CB2 2QQ
United Kingdom
pjah2@cam.ac.uk
Monisha Kumar, MD
Department of Neurology
Perelman School of Medicine, University of Pennsylvania
3 West Gates
3400 Spruce Street
Philadelphia, PA, 19104
USA
monisha.kumar@uphs.upenn.edu
Molly McNett, RN, PhD
Director, Nursing Research
The MetroHealth System
2500 MetroHealth Drive
Cleveland, OH 44109
USA
mmcnett@metrohealth.org
Chad Miller, MD
Division of Cerebrovascular Diseases and Neurocritical Care
The Ohio State University
395 W. 12th Ave, 7th Floor
Columbus, OH 43210
ChadM.Miller@osumc.edu
Andrew Naidech, MD, MSPH
Department of Neurology
Northwestern University Feinberg SOM 710
N Lake Shore Drive, 11th floor
Chicago, IL 60611
ANaidech@nmff.org
Mauro Oddo, MD
Department of Intensive Care Medicine
CHUV University Hospital, BH 08-623
Faculty of Biology and Medicine University of Lausanne
1011 Lausanne, Switzerland
Mauro.Oddo@chuv.ch
DaiWai Olson, RN, PhD
Associate Professor of Neurology, Neurotherapeutics and Neurosurgery
University of Texas Southwestern
5323 Harry Hines Blvd
Dallas, TX 75390-8897
USA
daiwai.olson@utsouthwestern.edu
Kristine O’Phelan M.D
Director of Neurocritical Care
Associate Professor, Department of Neurology
University of Miami, Miller School of Medicine
JMH, 1611 NW 12th Ave, Suite 405
Miami, FL, 33136
USA
kophelan@med.miami.edu
Javier Provencio, MD
Associate Professor of Medicine
Cerebrovascular Center and Neuroinflammation Research Center
Lerner College of Medicine
Cleveland Clinic
9500 Euclid Ave, NC30
Cleveland, OH 44195
USA
provenj@ccf.org
Corina Puppo, MD
Assistant Professor, Intensive Care Unit
Hospital de Clinicas, Universidad de la República,
Montevideo
Uruguay
coripuppo@gmail.com
Richard Riker, MD
Critical Care Medicine
Maine Medical Center
22 Bramhall Street
Portland, Maine 04102-3175
USA
RRiker@cmamaine.com
Claudia Robertson, MD
Department of Neurosurgery
Medical Director of Center for Neurosurgical Intensive Care
Ben Taub Hospital
Baylor College of Medicine
1504 Taub Loop, Houston, TX 77030
USA
claudiar@bcm.tmc.edu
J. Michael Schmidt, PhD, MSc
Director of Neuro-ICU Monitoring and Informatics
Columbia University College of Physicians and Surgeons
Milstein Hospital 8 Garden South, Suite 331
177 Fort Washington Avenue
New York, NY 10032
USA
mjs2134@columbia.edu
Fabio Taccone, MD
Department of Intensive Care, Laboratoire de Recherche Experimentale
Erasme Hospital
Route de Lennik, 808
1070 Brussels
Belgium
ftaccone@ulb.ac.be
Rights and permissions
About this article
Cite this article
Badjatia, N., Vespa, P. & And the Participants of the International Multi-disciplinary Consensus Conference on Multimodality Monitoring. Monitoring Nutrition and Glucose in Acute Brain Injury. Neurocrit Care 21 (Suppl 2), 159–167 (2014). https://doi.org/10.1007/s12028-014-0036-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-014-0036-2