Abstract
Background
Traumatic brain injury (TBI) is associated with a systemic hyperadrenergic state. Through activation of beta adrenoreceptors, catecholamines may induce hypermetabolism and increase both cardiac and cerebral oxygen demands. We conducted a systematic review to appraise the available evidence examining the safety and efficacy of beta blockers in patients with acute TBI.
Methods
We systematically searched CENTRAL, MEDLINE, EMBASE and the reference lists of relevant articles from database inception until March 19, 2013. The outcomes assessed were in-hospital mortality, functional outcome and quality of life. Common adverse effects of beta blockers were examined including clinically significant hypotension, bradycardia, bronchospasm and congestive heart failure. Data on study outcomes and quality were abstracted in duplicate. The results were summarized descriptively and quantitatively.
Results
One randomized controlled trial was found with a high risk of bias. Eight retrospective cohort studies were found with a moderate risk of bias; however, only four of these studies were identified as unique after excluding overlapping cases. The cohort studies reported mortality outcomes; however, none of these included studies assessed functional outcomes or quality of life. Meta-analysis on the cohort studies (n = 4,782 patients) demonstrated that exposure to beta blockers after TBI was associated with a reduction in the adjusted odds of in-hospital mortality by 65 % (pooled adjusted odds ratio 0.35; 95 % CI 0.27–0.45).
Conclusions
The current body of evidence is suggestive of a benefit of beta blockers following TBI. However, methodologically sound randomized controlled trials are indicated to confirm the efficacy of beta blockers in patients with TBI.
Similar content being viewed by others
Introduction
Traumatic brain injury (TBI) is a leading cause of mortality and morbidity worldwide. It is a major health and socioeconomic problem that results in an estimated 1.5 million injuries, more than 50,000 deaths, and the loss of over US $60 billion per year in the USA alone [1–4].
Because the primary injury cannot be reversed, the mainstay of TBI management to improve neurological outcome is the prevention of secondary injury. Currently, there is no targeted pharmacological treatment that effectively limits the progression of secondary injury and thereby improves the outcome [5]. However, extensive research focusing on the mechanisms of secondary insult following TBI has recently highlighted numerous potential targets for intervention.
TBI has been shown to be associated with a systemic hyperadrenergic state [6]. Through activation of beta adrenoreceptors, catecholamines may induce hypermetabolism and increase both cardiac and cerebral oxygen demands [6–8]. There are animal data that suggest potential neuroprotective effects of beta blockers by improving surrogate immunohistochemical markers of cerebral perfusion and decreasing cerebral oxygen demand, as observed with positron emission tomography [9–11]. In addition, beta blockers have been associated with beneficial cardioprotective and metabolic effects and improved outcomes in burn patients who have a similar hyperadrenergic state [12, 13]. A component of the Lund protocol to manage TBI that has been popular in Sweden is the use of the beta blocker metoprolol, in addition to other agents including an alpha-2 agonist and an angiotensin II inhibitor [14, 15]. These treatments are thought to decrease the intracapillary hydrostatic pressure and subsequently decrease the vasogenic edema that often complicates TBI. This protocol was derived from experimental animal studies and the physiological principles of cerebral autoregulation [14, 16].
Beta blockers have shown promising results in animal studies, case series and historical control studies in addition to multiple recent cohort studies [17, 18]. Therefore, a review of high-quality clinical studies examining the efficacy and safety of beta blockers after TBI is needed. The objectives of this review were as follows: (1) to determine the efficacy of beta blockers in reducing the mortality of hospitalized TBI patients; (2) to determine the efficacy of beta blockers in improving the functional outcomes and the quality of life of TBI patients; and (3) to determine the safety profile of beta blockers in the acute TBI population.
Methods
Types of Studies
We recognize that randomized controlled trials (RCTs) specifically designed to compare beta blockers against placebo in patients with TBI may not exist because beta blockers are medications that are used primarily for cardiovascular indications. Regardless, such trials were sought out so that they may be included in this review. Because of the high likelihood of a lack of RCTs, we also included quasi-randomized and non-randomized controlled trials, as well as cohort studies (prospective and retrospective) comparing TBI patients who received beta blockers after injury to those who did not. Other non-randomized study designs were excluded. Among the included studies, RCTs and observational studies were analyzed separately, as a direct comparison between the estimates of observational studies and RCTs can be misleading.
Types of Participants
We included studies that incorporated adult patients hospitalized with acute TBI.
Types of Interventions
All types, doses and routes of administration of beta blockers were included, provided they were given during the acute hospital stay and continued for any duration of time. The comparison group may have received either placebo or no treatment.
Types of Outcome Measures
The primary outcome of this review was in-hospital mortality. The secondary outcomes considered were as follows: (1) functional outcome at the latest time measured, as assessed using scales such as the Glasgow Outcome Score (GOS) scale, Extended Glasgow Outcome Score (GOSE) scale, Functional Independence Measure (FIM), or Disability Rating Scale (DRS); (2) quality of life at the latest time measured, as measured using standardized scales; (3) the safety of beta blockers use after TBI as assessed by the rate of common adverse effects of beta blockers such as clinically significant hypotension (i.e., systolic blood pressure <90 mm Hg, which requires fluid resuscitation, discontinuation of the study drug, an inotropic agent), clinically significant bradycardia (i.e., bradycardia requiring a temporary pacemaker, a sympathomimetic agent, atropine or discontinuation of the study drug), bronchospasm (i.e., bronchospasm requiring discontinuation of the beta blocker or use of a bronchodilator) and congestive heart failure.
Search Methods for Identification of Studies
We searched MEDLINE (from 1950 to March 19, 2013), EMBASE (from 1980 to March 19, 2013) and Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 2, 2013). The search was not restricted by date, language or publication status. The search strategy was based on the MEDLINE search strategy and was modified as necessary for the other databases (Supplementary Appendix). In addition, we searched the reference lists of relevant articles and the following registers to identify ongoing trials:
-
Clinical Trials (http://www.clinicaltrials.gov);
-
Current Controlled Trials (http://www.controlled-trials.com/mrct).
Data Collection and Analysis
Two of the review authors (AA and VM) independently examined all of the abstracts of the studies identified by our search and determined the eligibility of each study. Any disagreements were resolved by consensus. We scanned the titles and abstracts of every record retrieved to determine which of the studies should be assessed further. If it was clear from the title and abstract that the article was irrelevant, the article was rejected. The full texts of the remaining articles were then retrieved. The reference lists of the retrieved articles were also searched for additional citations.
Data abstraction forms were created and used to collect the relevant data from the included studies (Supplementary Appendix). Two of the review authors (AA and VM) independently extracted data on patients, methods, interventions (or exposures in the cohort studies), outcomes and results.
Two of the review authors (AA and VM) independently assessed the risk of bias for each included study. Any disagreement was resolved through discussion and consensus. Each included study was classified as a RCT or a cohort study, and the risk of bias was assessed differently for each type of study. For RCTs, we used the Cochrane Collaboration’s tool for assessing risk of bias [5] according to the following domains: sequence generation, allocation concealment, blinding of outcomes, incomplete outcome data, selective outcome reporting and baseline imbalances. For the cohort studies, selection of the exposed and non-exposed cohorts, the comparability of the cohorts, the assessment of the outcomes and the adequacy of follow-up were addressed. The Newcastle–Ottawa Scale (NOS) was used for assessing the risk of bias in the cohort studies (Supplementary Appendix) [20]. The scale was modified to include a number of TBI prognostic variables [age, pupillary reactivity and Glasgow Coma Scale (GCS) Score] under the comparability category and therefore allowed the reviewers to optimize the applicability of the scale to the TBI cohort studies. Our selection for these prognostic variables was based on the International Mission for Prognosis and Analysis of Clinical Trials (IMPACT) core prognostic model [21]. When considering comparability in the NOS, we assessed whether these important variables were adjusted for in the analysis. One to three points were awarded if age, the GCS motor score and/or the pupillary reaction were adjusted for by the study.
We calculated the odds ratio (OR) to measure the treatment effect for the dichotomous outcomes with corresponding 95 % confidence intervals (CI). Pooling of overall estimates of effect was performed using generic inverse variance weighting methods. Using these methods, each study estimate of the relative treatment is given a weight that is equal to the inverse of the variance of the effect estimate (i.e., one divided by the standard error squared). Clinical heterogeneity across the studies was assessed by examining the details of the subjects, the baseline data, and the interventions and the outcomes to determine whether the studies were sufficiently similar. Statistical heterogeneity was determined using the I 2 statistic and the chi-square test. There were too few studies to use a funnel plot to assess for reporting bias.
We used the Review Manager 5.1 software (RevMan 5.1, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012) to carry out a quantitative analysis. We performed a meta-analysis using a fixed-effect model because there was no evidence of significant clinical or statistical heterogeneity between the studies.
Results
Description of Studies
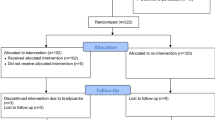
A total of 5,298 potentially relevant citations were screened for retrieval. 363 duplicates were excluded. A total of 4,911 were excluded after scanning the titles and/or abstracts because they did not meet our inclusion criteria (Fig. 1). A total of 24 citations were retrieved for detailed evaluation of the full text articles. Fifteen of those citations were excluded because they were case series, historical control studies or the measured outcomes were different than our set criteria for primary or secondary outcomes (further details are in the Supplementary Appendix).
After applying our selection criteria, we identified one RCT (by Cruickshank et al.) and eight retrospective cohort studies (Fig. 1) [22–30]. The cohort studies by Salim et al. [23], Inaba et al. [25], Hadjizacharia et al. [26] and Bukur et al. [29] appear to originate from the same cohort and were conducted mostly by the same group of investigators. The Salim et al. study sample appears to be a subgroup of the Inaba et al. but with a different analysis plan and objectives [23, 25]. The main objective of the Salim et al. cohort study was to investigate the relationship between troponin elevation and the outcome of severe TBI [23]. Similarly, the Hadjizachria et al. and the Bukur et al. cohorts appear to overlap with the Inaba et al. cohort. The primary objective of the Hadjizachria et al. study was to determine the association between atrial arrhythmias and the outcomes of trauma patients, while the Bukur et al. study evaluated the association between beta blockers and the outcomes of TBI across different racial groups [26, 29]. Therefore, we included only the Inaba et al. study and excluded the Salim et al., Hajizacharia et al. and Bukur et al. studies, because the Inaba et al. cohort was more representative of the general TBI population and the primary objective of the Inaba et al. study addressed the same question as our review. A similar overlapping case was found between the Cotton et al. and the Riordan et al. cohorts [24, 28]. We elected to include the Cotton et al. study and exclude the Riordan et al. study because the primary focus of the Riordan et al. study was to examine the relationship between beta blocker exposure and the outcome of a subgroup of TBI population who had early cardiac uncoupling [24]. Cotton et al. on the other hand included a sample that was more representative of the general TBI population [28]. Hence, the eight cohort studies that we reviewed constituted four unique cohorts.
Two ongoing trials were also identified. Ley et al. (ClinicalTrials.gov, NCT01202110) are conducting a phase II, dose escalation, single-center study on the effects of early propranolol on heart rate, blood pressure and cerebral perfusion pressure in subjects with moderate to severe TBI. Patel et al. (ClinicalTrials.gov, NCT01322048) are recruiting patients with acute severe TBI for a pilot RCT comparing propranolol and clonidine treatments versus a placebo. The primary outcome for this trial is ventilator-free days.
Included Studies
Descriptive statistics were extracted from the RCT by Cruickshank et al. and each of the eight cohort studies (Tables 1 and 2, a more detailed description is provided in the Supplementary Appendix). However, only data from the four unique cohort studies were used for the meta-analysis (Fig. 2).
The Cruickshank et al. study was a double-blinded placebo-controlled trial, published in 1987, that examined the safety and impact of atenolol on cardiac morbidity of patients with acute TBI [27]. This trial included patients aged 11–70 years old, with a primary diagnosis of acute TBI, and were admitted to the intensive care or neurosurgical unit of one of the four study centers in three European countries [27]. The study drug was initiated immediately after hemodynamic stabilization (mean time was 20.2 h following injury) [27].
The cohort studies included only hospitalized adult patients with TBI as defined by the head Abbreviated Injury Scale (AIS) score or by using the International Statistical Classification of Diseases, Ninth Revision (ICD-9CM) code for blunt TBI [22–26, 28–30]. The exposures in the included studies were defined as any beta blocker agent, regardless of dose or route of administration; however, all beta blockers were initiated during the acute hospital stay following TBI and continued for a variable length of time. The four unique cohort studies included a total of 4,782 patients. All of the cohort studies were conducted in the USA and were published between 2007 and 2012. None of the included studies declared sponsorship [22–30].
Risk of Bias in the Included Studies
The RCT by Cruickshank et al. had a high risk of bias because of a small sample size (n = 114), an unclear randomization and allocation concealment method, and incomplete outcome data (Table 3).
The risk of bias assessment of the included cohort studies was carried out using a modified NOS (Supplementary Appendix). Each one of the four unique cohort studies (Arbabi et al., Cotton et al., Inaba et al. and Schroeppel et al.) had a moderate risk of bias and reached 6–7 out of 9 points (Table 4).
Outcome Assessment
In-hospital mortality was assessed by all cohort studies but not by the RCT [22, 25, 28, 30]. None of the included studies examined functional outcome or quality of life measures.
The findings of the cohort studies are summarized in Table 2. All of the cohort studies demonstrated that beta blocker exposure after TBI was associated with older age, higher comorbidity burden and more severe injuries. The investigators of these studies attempted to adjust for potential confounding variables (Table 2). In adjusted analyses, all of the four unique cohort studies showed that beta blocker exposure following TBI was associated with reduced in-hospital mortality [22, 25, 28, 30].
Meta-analysis of the four unique cohort studies (Fig. 2) showed that exposure to beta blockers after TBI was associated with a reduction in the adjusted odds of in-hospital mortality by 65 % (pooled OR 0.35; 95 % CI 0.27–0.45; p < 0.00001, I 2 = 0 %).
Salim et al. suggested that beta blocker therapy was associated with a larger survival advantage among the subgroup of patients with isolated blunt TBI who had elevated troponin levels at some point during the first 48 h of their hospital stay [23]. None of the included cohort studies adequately described the different subgroups of TBI (mild, moderate and severe) to allow for a subgroup analysis of the relationship between beta blocker therapy and hospital mortality of the different TBI severity subgroups.
The only study that assessed for potential adverse events associated with beta blocker therapy in TBI population was the RCT by Cruickshank et al. (Table 1) [27]. Compared to the placebo group, there was a lower proportion of patients with abnormally high CK-MB level (2/27 vs. 9/30, respectively, p = 0.05) and a lower incidence of supraventricular tachycardia in the atenolol group (6/46 vs. 28/49, p < 0.0001). In addition, there was no significant difference between both groups in terms of the incidence of the other outcomes including hypotension, bradycardia, congestive heart failure and bronchospasm (Table 1) [27].
Discussion
Our review summarizes the mounting clinical evidence suggesting the safety and benefit of beta blocker therapy in patients with acute TBI. This meta-analysis suggests that beta blocker administration following acute TBI was associated with lower in-hospital mortality. There are no data regarding impact on functional outcomes or quality of life measures. Safety data from one study revealed no increase in adverse events in beta blockers group.
Beta blocker therapy in acute TBI is appealing, as an increasing body of physiological literature supports the hypothesis that beta blockers might be beneficial following TBI [7–10, 31–35]. Among the proposed mechanisms of benefit is the reduction in cerebral metabolic rate and oxygen demands as protective mechanism for the acutely injured and highly vulnerable parts of the brain [9, 10]. Systemically, beta blockers might also protect other end organs that are prone to damage by the TBI-induced catecholamine surge [31, 33, 36, 37]. In addition, a group from Lund, Sweden, has long advocated for the use of a management protocol for severe TBI based on the physiological principles of cerebral autoregulation, which includes the administration of the beta blocker metoprolol, to decrease the intracapillary hydrostatic pressure and thereby limit the formation of brain edema. [14]. However, our review indicates the lack of evidence from prospective studies to support this management protocol, although multiple case series and historical control studies have suggested a significant reduction in TBI mortality after the introduction of this protocol [15, 16, 38, 39]. The use of a historical control group fails to account for multiple important confounders including the change in the quality of general care of the critically ill patients over the last few decades. Thus, the Lund protocol, although promising, has not gained wide acceptance, and it needs to be evaluated by well-designed prospective studies.
In this systematic review, we used a very sensitive search strategy to identify all relevant observational studies and controlled trials without any restriction by date, language or publication status. In addition, two reviewers independently applied the inclusion criteria and assessed the risk of bias of the included studies using well-structured and validated tools.
The evidence supporting the use of beta blockers in TBI is certainly limited by the lack of well-designed controlled trials that address critically important outcomes in TBI population (e.g., mortality, functional and quality of life outcomes), and the fact that most relevant studies were retrospective in nature. The limitations of retrospective cohort studies in this area include the possibility of a survivor-treatment bias. Patients in the beta blockers group may have simply lived long enough to receive beta blockers, whereas those in the comparison group may have died prior to having the opportunity to receive beta blockers. Some of the included studies tried to address this bias. Cotton et al. attempted to address this bias by excluding patients with a length of stay <4 days, which implies the exclusion of early deaths [28]. The survival advantage of beta blockers, after excluding these early deaths, remains statistically significant [28]. Schroeppel et al. showed that the survival advantage was smaller, but still significant, after excluding deaths within the first 24 h and non-significant after excluding deaths within 48 h post-injury [22]. Although the results from Cotton et al. study and the sensitivity analyses of Schreoppel et al. study appear reassuring, the optimal way to address the survivor-treatment bias will be to conduct a RCT. The included studies are also limited by the inclusion of multiple beta blocking agents with variable affinity to different types of beta receptors, the different dosing regimens, and the variable timing and duration of beta blocker administration. Nevertheless, the relative homogeneity, consistency and magnitude of the survival advantage shown in the meta-analysis, among the older, more severely injured and chronically ill patients who received beta blockers, provide an argument for designing RCTs in the future to address this important research question.
Issues that should be addressed by RCTs in the future include the safety of beta blockers in the acute TBI population, the optimal beta blocker agent and dosing regimen for this indication, the impact of beta blockers use on the long-term functional and quality of life outcomes of TBI patients, and whether beta blockers are more beneficial in specific subgroups of TBI patients.
Conclusions
The current body of evidence is suggestive of a benefit of beta blocker therapy in patients hospitalized with acute TBI. Prior to making any practice recommendations, well-designed randomized controlled trials are needed to better evaluate the safety and impact of this promising intervention on long-term mortality, functional outcome and quality of life of patients following TBI.
References
Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–41.
Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehab. 2006;21:375–8.
Shackford SR, Mackersie RC, Holbrook TL, Davis JW, Hollingsworth-Fridlund P, Hoyt DB, Wolf PL. The epidemiology of traumatic death. A population-based analysis. Arch Surg. 1993;128:571–5.
Thurman D, Guerrero J. Trends in hospitalization associated with traumatic brain injury. JAMA. 1999;282:954–7.
Park E, Bell JD, Baker AJ. Traumatic brain injury: can the consequences be stopped? CMAJ. 2008;178:1163–70.
Neil-Dwyer G, Cruickshank JM, Doshi R. The stress response in subarachnoid haemorrhage and head injury. Acta Neurochir Suppl. 1990;47:102–10.
Hortnagl H, Hammerle AF, Hackl JM, Brucke T, Rumpl E, Hortnagl H. The activity of the sympathetic nervous system following severe head injury. Intensive Care Med. 1980;6:167–9.
McLeod AA, Neil-Dwyer G, Meyer CH, Richardson PL, Cruickshank J, Bartlett J. Cardiac sequelae of acute head injury. Br Heart J. 1982;47:221–6.
Ley EJ, Scehnet J, Park R, Schroff S, Dagliyan G, Conti PS, Margulies DR, Salim A. The in vivo effect of propranolol on cerebral perfusion and hypoxia after traumatic brain injury. J Trauma. 2009;66:154–9; discussion 159–161.
Ley EJ, Park R, Dagliyan G, Palestrant D, Miller CM, Conti PS, Margulies DR, Salim A. In vivo effect of propranolol dose and timing on cerebral perfusion after traumatic brain injury. J Trauma. 2010;68:353–6.
Ley EJ, Clond MA, Bukur M, Park R, Chervonski M, Dagliyan G, Margulies DR, Lyden PD, Conti PS, Salim A. Beta-adrenergic receptor inhibition affects cerebral glucose metabolism, motor performance, and inflammatory response after traumatic brain injury. J Trauma Acute Care Surg. 2012;73(1):33–40.
Herndon DN, Hart DW, Wolf SE, Chinkes DL, Wolfe RR. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223–9.
Arbabi S, Ahrns KS, Wahl WL, Hemmila MR, Wang SC, Brandt MM, Taheri PA. Beta-blocker use is associated with improved outcomes in adult burn patients. J Trauma. 2004;56:265–9; discussion 269–271.
Asgeirsson B, Grande PO, Nordstrom CH. A new therapy of post-trauma brain oedema based on haemodynamic principles for brain volume regulation. Intensive Care Med. 1994;20:260–7.
Naredi S, Eden E, Zall S, Stephensen H, Rydenhag B. A standardized neurosurgical neurointensive therapy directed toward vasogenic edema after severe traumatic brain injury: clinical results. Intensive Care Med. 1998;24:446–51.
Grande PO, Nordstrom CH, Asgeirsson B. Current alternative therapy of post-traumatic brain edema. Nord Med. 1994;109:157–9.
Tran TY, Dunne IE, German JW. Beta blockers exposure and traumatic brain injury: a literature review. Neurosurg Focus. 2008;25:E8.
Heffernan DS, Inaba K, Arbabi S, Cotton BA. Sympathetic hyperactivity after traumatic brain injury and the role of beta-blocker therapy. J Trauma. 2010;69:1602–9.
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf.
Steyerberg EW, Mushkudiani N, Perel P, et al. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008;5(8):e165; discussion e165.
Schroeppel TJ, Fischer PE, Zarzaur BL, Magnotti LJ, Clement LP, Fabian TC, Croce MA. Beta-adrenergic blockade and traumatic brain injury: protective? J Trauma. 2010;69:776–80.
Salim A, Hadjizacharia P, Brown C, Inaba K, Teixeira PGR, Chan L, Rhee P, Demetriades D. Significance of troponin elevation after severe traumatic brain injury. J Trauma. 2008;64:46–52.
Riordan WP Jr, Cotton BA, Norris PR, Waitman LR, Jenkins JM, Morris JA Jr. Beta-blocker exposure in patients with severe traumatic brain injury (TBI) and cardiac uncoupling. J Trauma. 2007;63:503–10; discussion 510–501.
Inaba K, Teixeira PGR, David J-S, Chan LS, Salim A, Brown C, Browder T, Beale E, Rhee P, Demetriades D. Beta-blockers in isolated blunt head injury. J Am Coll Surg. 2008;206:432–8.
Hadjizacharia P, O’Keeffe T, Brown CVR, Inaba K, Salim A, Chan LS, Demetriades D, Rhee P. Incidence, risk factors, and outcomes for atrial arrhythmias in trauma patients. Am Surg. 2011;77:634–9.
Cruickshank JM, Neil-Dwyer G, Degaute JP, Hayes Y, Kuurne T, Kytta J, Vincent JL, Carruthers ME, Patel S. Reduction of stress/catecholamine-induced cardiac necrosis by beta 1-selective blockade. Lancet. 1987;2:585–9.
Cotton BA, Snodgrass KB, Fleming SB, Carpenter RO, Kemp CD, Arbogast PG, Morris JA Jr. Beta-blocker exposure is associated with improved survival after severe traumatic brain injury. J Trauma. 2007;62:26–33; discussion 33–25.
Bukur M, Mohseni S, Ley E, Salim A, Margulies D, Talving P, Demetriades D, Inaba K. Efficacy of beta-blockade after isolated blunt head injury: does race matter? [Erratum appears in J Trauma Acute Care Surg. 2012 Jun;72(6):1725 Note: Mosheni, Shahin [corrected to Mohseni, Shahin]]. J Trauma Acute Care Surg. 2012;72:1013–18.
Arbabi S, Campion EM, Hemmila MR, Barker M, Dimo M, Ahrns KS, Niederbichler AD, Ipaktchi K, Wahl WL. Beta-blocker use is associated with improved outcomes in adult trauma patients. J Trauma. 2007;62:56–61; discussion 61–52.
Chiolero RL, Breitenstein E, Thorin D, Christin L, de Tribolet N, Freeman J, Jequier E, Schutz Y. Effects of propranolol on resting metabolic rate after severe head injury. Crit Care Med. 1989;17:328–34.
Zlotnik A, Klin Y, Gruenbaum BF, Gruenbaum SE, Ohayon S, Leibowitz A, Kotz R, Dubilet M, Boyko M, Shapira Y, Teichberg VI. Beta2 Adrenergic-mediated reduction of blood glutamate levels and improved neurological outcome after traumatic brain injury in rats. J Neurosurg Anesthesiol. 2012;24:30–8.
Larson B, Beal A, Ahari A, Russell S, Freeman K. Beta-adrenergic blockade prevents myocardial oxidative stress due to traumatic brain injury. Ann Emerg Med. 2009;1:S142–3.
Liu MY. Protective effects of propranolol on experimentally head-injured mouse brains. J Formos Med Assoc. 1995;94:386–90.
Larson BE, Stockwell DW, Boas S, Andrews T, Wellman GC, Lockette W, Freeman K. Cardiac reactive oxygen species after traumatic brain injury. J Surg Res. 2012;173:e73–81.
Leitman IM. Understanding the brain-heart axis in neurological trauma. J Surg Res. 2012;173:e33–5.
Hortnagl H, Hammerle AF, Hackl JM, Brucke T, Rumpl E. The activity of the sympathetic nervous system following severe head injury. Intensive Care Med. 1980;6:167–9.
Naredi S, Olivecrona M, Lindgren C, Ostlund AL, Grande PO, Koskinen LOD. An outcome study of severe traumatic head injury using the “Lund therapy” with low-dose prostacyclin. Acta Anaesthesiol Scand. 2001;45:402–6.
Eker C, Asgeirsson B, Grande PO, Schalen W, Nordstrom CH. Improved outcome after severe head injury with a new therapy based on principles for brain volume regulation and preserved microcirculation. Crit Care Med. 1998;26:1881–6.
Acknowledgments
We would like to thank Elizabeth Uleryk for her assistance in designing the literature search strategy. This work was supported in part by funds from Physicians’ Services Inc. Foundation (to A.A.) and a Canada Research Chair Program in Systems of Trauma Care (to A.B.N.).
Conflict of interest
No competing financial interests exist.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alali, A.S., McCredie, V.A., Golan, E. et al. Beta Blockers for Acute Traumatic Brain Injury: A Systematic Review and Meta-analysis. Neurocrit Care 20, 514–523 (2014). https://doi.org/10.1007/s12028-013-9903-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-013-9903-5