Abstract
Background
Previous studies of glycemic control in non-neurologic ICU patients have shown conflicting results. The purpose was to investigate whether intensive insulin therapy (IIT) to keep blood glucose levels from 80 to 110 mg/dl or conventional treatment to keep levels less than 151 mg/dl was associated with a reduction of mortality and improved functional outcome in critically ill neurologic patients.
Methods
Within 24 h of ICU admission, mechanically ventilated adult neurologic patients were enrolled after written informed consent and randomized to intensive or conventional control of blood glucose levels with insulin. Primary outcome measure was death within 3 months. Secondary outcome measures included 90-day modified Rankin scale (mRS) score, ICU, and hospital LOS.
Results
81 patients were enrolled. The proportion of deaths was higher among IIT patients but this was not statistically significant (36 vs. 25%, P = 0.34). When good versus poor outcome at 3 months was dichotomized to mRS score 0–2 versus 3–6, respectively, there was no difference in outcome between the two groups (76.2 vs. 75% had a poor 3-month outcome, P = 1.0). There was also no difference in ICU or hospital LOS. Hypoglycemia (<60 mg/dl) and severe hypoglycemia (<40 mg/dl) were more common in the intensive arm (48 vs. 11%, P = 0.0006; and 4 vs. 0%, P = 0.5, respectively).
Conclusion
There was no benefit to IIT in this small critically ill neurologic population. This is the first glycemic control study to specifically examine both critically ill stroke and traumatic brain injury (TBI) patients and functional outcome. Given these results, IIT cannot be recommended over conventional control.
Similar content being viewed by others
Introduction
Hyperglycemia is a common occurrence in critically ill patients. In animal studies of ischemic and hemorrhagic stroke, hyperglycemia has been associated with increased ischemic brain damage, edema, and peri-lesional cell death [1, 2]. Increased glucose concentration in the ischemic region is thought to cause increased edema, weakened blood vessels, and higher lactate concentration and acidosis thereby increasing generation of free radicals that damage neurons. Early hyperglycemia in rats with moderate traumatic brain injury (TBI) was associated with a larger contusion area and increased inflammation [3].
Several human studies have examined hyperglycemia in stroke and TBI. Admission hyperglycemia in the TOAST trial was associated with a worse outcome at 3 months after stroke onset [4]. The PROACT II urokinase and NINDS rt-PA trials revealed higher glucose (particularly over 200 mg/dl) was associated with lower frequency of neurologic improvement and higher risk for symptomatic intracerebral hemorrhage [5, 6]. Other stroke studies also confirm worse functional outcome with persistent hyperglycemia [7, 8]. In moderate to severe TBI studies, higher initial and postoperative glucose levels were associated with high intracerebral lactate levels and worse outcome, especially with a blood glucose level of greater than 160–200 mg/dl [9–12]. The question of whether hyperglycemia is simply a marker of more severe disease or actually causes worse outcome has not been definitively answered. However, the impact of interventions such as more aggressive control of blood sugars with intensive insulin therapy (IIT) has been studied.
IIT to keep blood glucose between 80 and 110 mg/dl was shown to reduce mortality, morbidity, and intensive care unit (ICU) length of stay (LOS) in adult surgical ICU patients [13]. IIT patients were compared with those randomized to conventional treatment where an infusion of insulin was given to maintain a blood glucose level of 180 to 215 mg/dl. Overall in-hospital mortality rates were lower in the IIT group at 7.2% compared with 10.9% (P < 0.01). Bloodstream infections and critical illness polyneuropathy were less frequent in the IIT group. In a post-hoc analysis, IIT was associated with a better outcome when compared with intermediate blood glucose control of 110 to 150 mg/dl [14]. Most of the patients, however, had cardiac surgery (63%) and only 4% had a neurologic diagnosis, TBI, or neurosurgery [13]. Although there was a lower mortality rate in the IIT group (18.2% compared with 23.3%), the numbers with neurologic disease were too small to reach statistical significance. Neurologic outcomes in terms of functional level after hospital discharge were not studied.
The same Belgian group studied their medical ICU patients but found no difference in mortality with IIT. However, they did find improved morbidities and length of stay. Mortality was improved in the subset of patients who stayed in the ICU for 3 or more days but this could not be predicted very well on admission [15].
In a recent study of over 6,000 medical and surgical ICU patients, very different results were obtained with a comparison of target blood glucose 81–108 mg/dl with target 180 mg/dl or less [16]. There was a higher 90 day mortality rate in the intensive group at 27.5% compared with 24.9% (P = 0.02). There were no differences in median ICU or hospital LOS or number of days of mechanical ventilation. The number of neurologic patients was not mentioned. From subsequent personal communication with the lead author, up to 6% of patients were noted to have a TBI but no other information was available. There are clearly conflicting data on how to manage blood glucose in medical and surgical patients in the ICU and the optimal blood glucose management for critically ill neurologic patients remains to be determined.
The purpose of our randomized single center study was to determine if IIT to keep blood glucose levels between 80 and 110 mg/dl as compared to conventional treatment to keep levels less than 151 mg/dl would be associated with a reduction of mortality and improved neurologic functional outcome in critically ill neurologic patients.
Methods
The study was approved by The Queen’s Medical Center (QMC), Honolulu, Hawaii, Institutional Review Board. Within 24 h of admission to The QMC Neuroscience Intensive Care Unit (NSICU), mechanically ventilated neurologic patients expected to have an ICU LOS of at least 3 days were identified and written informed consent was obtained from the patient or the patient’s surrogate decision-maker prior to randomization. The inclusion criterion of predicted ICU LOS of at least 3 days was based on previous studies’ findings of specific benefit of IIT for those with this longer ICU LOS [13, 15]. Patients enrolled in this study had to be at least 18 years of age and have a neurologic admission diagnosis of ischemic stroke, intracerebral hemorrhage, subarachnoid hemorrhage, traumatic brain or spinal cord injury, meningoencephalitis, status epilepticus, brain or spinal cord tumor, myasthenia gravis or Guillain-Barré syndrome. Exclusion criteria included current enrollment in another research study, pregnancy or nursing, or a time lapse of greater than 24 h since the patient’s admission to the NSICU. Patients were enrolled between September 2004 and July 2008.
Study Design
Once written informed consent was obtained, patients were randomized to either intensive control (range of 80–110 mg/dl) or to conventional control (≤150 mg/dl based on previous standard of care at QMC). Treatment assignments were kept in a sealed envelope to be opened after enrollment at which time clinical staff were aware of the assigned treatment arm.
If the patient was randomized to the intensive group, blood glucose levels were obtained via an arterial catheter, or fingerstick (capillary) testing if an arterial catheter was not available, every 1 h initially and reduced to every 2 h as levels stabilized. A continuous insulin infusion (100 units regular insulin in 100 milliliters of 0.9% sodium chloride solution) was delivered via a central or peripheral venous catheter and titrated for a goal blood sugar of 80 to 110 mg/dl. Glucommander software, a computer-directed algorithm, was used to calculate the rate and determine hourly adjustments of the continuous insulin infusion to keep blood sugars in this pre-specified range. The algorithm calculates the insulin infusion rate according to the formula: units per hour is equal to the current blood glucose level subtracted by 60 multiplied by a sensitivity factor (0.01 for those most sensitive to insulin, 0.02 if moderately sensitive, and 0.03 in those least sensitive to insulin) [17].
If the patient was randomized to the conventional treatment group, then the blood glucose was measured every 6 h either via an arterial line or fingerstick (capillary) testing. Treatment was initiated whenever the blood sugar level was greater than 150 mg/dl and a standardized regular insulin sliding scale was utilized starting with 3 units of subcutaneous regular insulin for a blood sugar of 151 to 200 mg/dl and increasing stepwise by 2 units for every 50 mg/dl higher range thereafter. If there were two documented blood sugars above 200 mg/dl, then the treating physician could opt to switch to a continuous insulin infusion that started at blood glucose of 151 mg/dl and then follow a written algorithm for titration with the same goal of less than 151 mg/dl.
Glucose level was primarily determined by a point-of-care glucometer. All patients were fed an enteral diet via a nasogastric or orogastric tube as soon as possible after NSICU admission unless an alternate regimen was deemed more appropriate by the treating physician. Information on the type and route of feeding was recorded throughout the hospital stay. For both the treatment groups, when the patient was discharged from the NSICU, conventional management of the blood sugar was performed with every 6-h blood sugar measurements via fingersticks utilizing the same regular insulin sliding scale as in the conventional arm whenever the blood sugar was greater than 150 mg/dl.
540 patients (270 per treatment group) were planned to be enrolled to have at least an 80% chance (power = 0.8) of detecting a 40% reduction in mortality between the two groups. This sample size took into account that some patients might need to be analyzed both together and separately from the rest of the study population due to death from family consensus decision to withdraw life support.
Data Collection
The patient’s gender, age, neurologic diagnosis, concurrent diagnoses, past medical history, and NSICU admission GCS score were recorded. To quantify function pre-hospitalization, modified Rankin scale (mRS) score was determined ranging from 0 for no symptoms to 5 for severe disability and bedridden.
All episodes of hypoglycemia (blood glucose <60 mg/dl) and severe hypoglycemia (<40 mg/dl) were recorded, and documentation was made as to whether the patient was symptomatic (e.g., alteration in mental status or seizure). All episodes of blood transfusions, bloodstream infections (growth on blood culture not from contaminant), and pneumonia (infiltrate on chest radiograph and growth on sputum culture) were recorded. Blood cultures and sputum cultures were obtained when the patient had a temperature greater than 101°F or at the discretion of the treating physician.
Information on number of days of mechanical ventilation, ICU stay, and hospital stay were collected. The patients were disconnected from the ventilator and extubated as soon as deemed safe by the treating physician. The patients were transferred out of the NSICU the day after extubation as long as they were considered by the treating physician to be hemodynamically and neurologically stable for transfer to a regular ward. Ninety days after the initial NSICU admission, mRS score was obtained based on a phone interview with the patient or patient’s caregiver by a research staff member blinded to the study treatment assignment. If the patient was still hospitalized at 90 days, then the mRS score was directly determined by the research staff member.
Outcome Measures
The primary outcome measure was death from any cause within 90 days after randomization. Predetermined secondary outcome measures were 90-day mRS score, LOS in the NSICU, hospital LOS, number of days on mechanical ventilation, number of hypoglycemic episodes, bloodstream infections, pneumonia, and red blood cell transfusions.
Statistical Analysis
Differences in baseline characteristics were analyzed using the student’s t test and Pearson’s χ2 test for continuous and categorical variables, respectively. Differences between the two intervention groups in the distribution of mRS score (operationalized as ≤2 vs. >2) and ICU and hospital mortality were analyzed using logistic regression with logistic regression treatment effect expressed as odds ratios. After adjusting for confounders, differences between the two intervention groups in hospital LOS, ICU LOS, and ventilator days, were analyzed using Gamma regression with Log link. Treatment effect in Gamma regression was expressed as ratios of means. All analyses were performed on an intention-to-treat basis. Data analysis was performed using SAS version 9.13.
Results
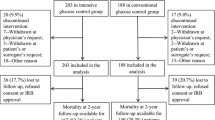
81 patients were enrolled (Fig. 1) before the study was halted due to the principal investigator’s re-location. 56 patients (69%) were male (Table 1). Mean and median ages were 51 with a range from 18 to 95 years of age. 38% of patients were Asian, 27% Pacific Islander, 25% Caucasian, 2% Hispanic, 1% African American, and 6% were of unknown ethnicity. Admission neurologic diagnoses included: 49% TBI, 35% stroke (22% intracerebral hemorrhage, 9% subarachnoid hemorrhage, and 4% ischemic stroke), 9% traumatic spinal cord injury, 5% status epilepticus, 1% meningoencephalitis, and 1% myasthenia gravis. The initial diet was started within 1 day of study enrollment and was either Glucerna®, Jevity®, or Peptamen® tube feeds in 87% of patients with only 10% of patients NPO at the start of the study.
Of the 81 patients enrolled, 45 were randomized to the intensive arm and 36 to the conventional arm (Fig. 1). There were no significant differences between the two groups in terms of gender, age, ethnicity, or admission neurologic diagnosis (Table 1). There were also no differences in past medical history between the two treatment groups (data not shown). Baseline mRS score did not differ between the two groups (80% of the intensive arm and 94% of the conventional arm had a mRS ≤2 (P = 0.10). 78% of patients had a mRS score of 0, i.e., completely normal neurologic function prior to this admission and this was similar between the two treatment arms. Glasgow Coma Scale on enrollment in the study was also similar between the two treatment groups at a mean of 6.8 vs. 6.9 (P = 0.84). Complete data were available for 91.4% of subjects. Seven of the 81 patients enrolled (three in the intensive arm and four in the conventional arm) were lost to follow-up.
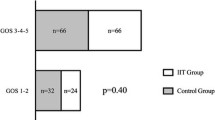
The primary outcome measure was death from any cause within 90 days after randomization. The proportion of deaths was higher among patients in the intensive arm but this was not statistically significant (36 vs. 25%, P = 0.34, Table 2; Fig. 2). Most common cause of death (in 21 patients, 84%) was neurologic deterioration with either progression to brain death or family decision to withdraw support. One patient died from cardiac arrest. Four deaths occurred after hospital discharge with etiology of brain death in one (in a long-term acute care facility) and etiology unavailable in the other three. The numbers were insufficient to analyze mortality rates in those deaths that were not due to family consensus decision to withdraw life support.
Median ICU LOS and hospital LOS were not significantly shorter in the intensive arm (Table 2). Median days on the ventilator were similar between the two groups (Table 2).
When good versus poor outcome at 3 months was dichotomized to mRS score 0–2 versus 3–6, respectively, there was no difference in outcome between the two groups (Table 2). Even when good outcome was expanded to include mRS of 3 (moderate disability requiring some help but able to walk without assistance), poor outcome of mRS >3 was still similar between the two treatment arms (64.3% of intensive arm compared with 59.4% of conventional arm, P = 0.81).
The blood glucose levels of conventional arm (80–150 mg/dl) patients were more frequently within target range than those of the intensive arm (80–110 mg/dl) (63.6 vs. 45.5% of the time, P = 0.001). This was expected given the narrower target range for the intensive arm. The blood glucose level was below the target range significantly more often in the intensive arm at 7.9% of the time versus 1.3% of the time (P < 0.0001) and above range significantly more often in the intensive arm at 46 vs. 35% of the time (P = 0.009). The mean average insulin infusion for those in the intensive arm was significantly higher at 2.39 units per hour (median 1.81, range 0.01–11.9) compared with 1.40 units per hour (median 0.84, range 0–6.2) in the conventional arm (P = 0.003).
Adverse Events
Hypoglycemic episodes were more likely to occur in the intensive arm with 21 patients (47%) experiencing hypoglycemia compared with four patients (11%) in the conventional arm (Table 2). Two patients (both in the intensive group) had a blood glucose level below 40 mg/dl. The mean average glucose level was significantly lower in the intensive arm at 112 mg/dl (median 109 mg/dl) compared with 143 mg/dl (median 140 mg/dl) (P < 0.0001 for the mean averages). The mean of the maximum glucose levels was not significantly higher in the conventional arm at 217 mg/dl versus 201 mg/dl (P = 0.22); however, the mean of the minimum glucose levels was significantly lower in the intensive arm at 62 versus 88 mg/dl (P < 0.0001). None of the hypoglycemic episodes were associated with mental status changes or seizures, although many of the patients already had a compromised mental status on study enrollment. Interestingly, none of the hypoglycemic episodes occurred while the patient was NPO.
Pneumonia was more common in the intensive arm but this was not statistically significant. There were six bloodstream infections, four of which were in the conventional arm (Table 2). There was also no difference in numbers of patients obtaining red blood cell transfusions (Table 2). The overall number of units transfused did not differ between the two groups with ratio of means estimated at 1.16 (95% CI 0.82–1.63, P = 0.41).
Group with Baseline mRS <3
When those patients with a baseline mRS score of greater than 2 were excluded from the analysis (removing 11 patients), there was still no significant difference in 90 day poor outcomes (mRS >2), 69.7% of intensive arm versus 76.7% of conventional arm (P = 0.58). In this sub-analysis of patients with a baseline mRS score of 2 or less, there was also no difference in deaths (P = 0.79), ICU LOS (P = 0.37), or hospital LOS (which approached but did not reach statistical significance, P = 0.06). Even when 90-day poor outcome was defined as mRS >3 and only patients with baseline mRS <3 were included, there was no difference in outcome between treatment groups (60.6% of intensive arm versus 60.0% of conventional arm).
Subgroup Analysis by Diagnosis
The two most common admission diagnoses were TBI (49%) and hemorrhagic and ischemic stroke (35%). When analyzing the data for just the subgroup with TBI, the results remained the same, with no difference in rate of poor outcome between the two treatment arms (Table 3). Deaths occurred in 36% of intensive arm patients and 33% of conventional arm patients (P = 1.0). Among the TBI patients, there was no difference in median ICU LOS, hospital LOS, or number of ventilator days between the two treatment groups. There was also no difference in number of red blood cell transfusions or rates of pneumonia. Hypoglycemic episodes were more frequent in the IIT group (62 vs. 11%, Table 3).
When only those with a diagnosis of stroke were analyzed (28 patients), there was no difference in rate of poor outcome (Table 3). There was a higher though non-significant rate of deaths in the intensive arm (50 vs. 21%, P = 0.24). The ICU LOS was shorter in the intensive arm but this did not reach statistical significance (Table 3). The total hospital LOS, however, was significantly shorter in the intensive arm (median of 16 vs. 26 days, P = 0.02). The median number of ventilator days in the intensive arm was shorter at 8 compared with 12 but this was not statistically significant. There were no differences between the two treatment groups in terms of hypoglycemic episodes, pneumonia, or red blood cell transfusions. The only other difference in this sub-analysis compared with the entire cohort is that the baseline mRS score was greater than 0 in 5 patients in the intensive arm and in none of the patients in the conventional arm (P = 0.04).
When only the TBI and stroke patients were included in the sub-analysis (68 patients), there was still no difference in 90 day outcomes between the two groups (72.7% of intensive versus 75% of conventional arm patients with mRS >2, P = 1). There was a non-significantly higher rate of deaths at 90 days in the intensive arm at 41.6 vs. 28.1%, P = 0.31).
Conclusion
There was no survival or outcome benefit to IIT over conventional insulin therapy in this small critically ill neurologic population. There was, in fact, a higher rate of hypoglycemia with IIT. Among stroke patients, however, there was a reduced total hospital LOS in those that received IIT. One explanation for this may be that there was a non-significantly higher rate of in-hospital deaths in the intensive arm of those with stroke so hospital LOS appeared shorter.
While our study’s findings differed from the overall results of the two large single-center trials [13, 15], they are similar to the results of the neurologic sub-group analysis of the former study [13] which showed no significant difference between treatment groups for in-hospital mortality. Our results were also similar to the more recent multi-center NICE-SUGAR trial where there were no differences in median ICU or hospital LOS or ventilator days [16]. Unlike the NICE-SUGAR trial, however, IIT was not associated with a significantly higher 90-day mortality rate.
Since our target glucose ranges for the two treatment arms were closer than the ranges in previous studies, it may have been more difficult to detect a difference in treatment outcomes. Unlike two previous single center studies [13, 15] that defined conventional treatment as a goal of 180 to 215 mg/dl, our study used a different target glucose level based on our previous standard of care of less than 151 mg/dl. Also, even though initially the conventional treatment patients were only monitored every 6 h in our study, several patients were transitioned to a continuous insulin infusion to keep the blood glucose less than 151 mg/dl. Once on a continuous infusion, blood glucose levels were monitored every 1–2 h just as in the intensive arm, possibly lessening the likelihood of finding a difference between the two treatment arms.
The target glucose range was only achieved in the intensive arm of our trial 46% of the time, mostly due to drops below 80 mg/dl. Previous studies did not give information on the efficacy of each of the treatment arms in achieving the goal blood glucose range. The surgical ICU trial, however, did report a mean morning blood glucose level of 103 ± 19 mg/dl in the IIT patients versus 153 ± 33 mg/dl in the conventional therapy patients [13]. The NICE-SUGAR trial [16] reported the mean time-weighted blood glucose level of 115 ± 18 mg/dl with IIT (higher than the upper limit goal of 108 mg/dl) versus 144 ± 23 mg/dl (within the target of ≤180 mg/dl), which is similar to our mean glucose level of 113 ± 9 mg/dl in the IIT group versus 142 ± 20 mg/dl in the conventional group. So it does not appear that there was a difference in actual blood sugars achieved in each treatment arm between our study and that of the NICE-SUGAR trial.
Hypoglycemic episodes were more frequent in the intensive arm of our study, but this was not dissimilar to hypoglycemia rates reported in earlier trials. Severe hypoglycemic episodes (<40 mg/dl) occurred in 5.2% of IIT versus 0.8% of conventional therapy patients in the surgical ICU trial [14] and occurred in a much higher 18.7 versus 3.1% in the follow-up medical ICU trial [15]. Rates in the NICE-SUGAR trial were 6.8% of IIT versus 0.5% in the conventional group [16]. This is similar to our study where 4.4% of IIT patients had a severe hypoglycemic episode (<40 mg/dl) versus 0% of the conventional patients. Blood glucose levels below 60 mg/dl were not reported in those other trials. In neurologic patients there may be more of a concern with having even low normal blood glucose levels and its impact on intracerebral glucose availability and metabolism and, ultimately, on neurologic recovery [18, 19]. Therefore, the fact that the mean of the minimum glucose levels in the IIT group was 62 mg/dl may be significant in a neurologic patient.
The main limitations of our study were the small number of patients enrolled and that we could not keep the treatment arm blinded from the treating staff once treatment was started. We did, however, keep the 90-day follow-up phone interviewer blinded as to the treatment given. Our study was limited to neurocritical care patients and cannot necessarily be extrapolated to other patient populations. Our results, though, are similar to the neurologic sub-group analysis of the single-center SICU trial [13], to the largest medical and surgical ICU trial to date on IIT [16], and to that of a meta-analysis which found no significant difference in mortality [20].
The question remains as to whether the lack of benefit of IIT is primarily due to the higher risk of hypoglycemia and if hypoglycemia could be reduced with better management strategies, would IIT then be beneficial to critically ill neurologic patients? If minimizing hypoglycemia is not an achievable goal, then perhaps a less “intensive” approach such as the less than 151 mg/dl goal used in the conventional arm would be more appropriate. A multicenter randomized trial would need to be done to best determine the optimal blood glucose management for critically ill neurologic patients.
In conclusion, there was no benefit to IIT in this small critically ill neurologic population in terms of mortality, morbidity, ICU LOS, or functional outcome. Previous studies of glycemic control in primarily non-neurologic ICU patients have shown conflicting results. This is the first glycemic control study to specifically examine both critically ill stroke and TBI patients and their functional outcome. Given these results, IIT cannot be recommended over conventional control.
References
Pulsinelli WA, Waldman S, Rawlinson D, Plum F. Moderate hyperglycemia augments ischemic brain damage: a neuropathologic study in the rat. Neurology. 1982;32:1239–46.
Song E, Chu K, Jeong S, et al. Hyperglycemia exacerbates brain edema and perihematomal cell death after intracerebral hemorrhage. Stroke. 2003;34:2215–20.
Kinoshita K, Kraydieh S, Alonso O, et al. Effect of posttraumatic hyperglycemia on contusion volume and neutrophil accumulation after moderate fluid-percussion brain injury in rats. J Neurotrauma. 2002;19:681–92.
Bruno A, Biller J, Adams HP Jr, et al. Acute blood glucose level and outcome from ischemic stroke. Trial of ORG 10172 in Acute Stroke Treatment (TOAST) Investigators. Neurology. 1999;52:280–4.
Kase CS, Furlan AJ, Wechsler LR, et al. Cerebral hemorrhage after intra-arterial thrombolysis for ischemic stroke: the PROACT II trial. Neurology. 2001;57:1603–10.
Bruno A, Levine SR, Frankel MR, et al. Admission glucose level and clinical outcomes in the NINDS rt-PA Stroke Trial. Neurology. 2002;59:669–74.
Els T, Klisch J, Orszagh M, et al. Hyperglycemia in patients with focal cerebral ischemia after intravenous thrombolysis: influence on clinical outcome and infarct size. Cerebrovasc Dis. 2002;13:89–94.
Baird TA, Parsons MW, Phanh T, et al. Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome. Stroke. 2003;34:2208–14.
Rovlias A, Kotsou S. The influence of hyperglycemia on neurological outcome in patients with severe head injury. Neurosurgery. 2000;46:335–42.
Lam AM, Winn HR, Cullen BF, Sundling N. Hyperglycemia and neurological outcome in patients with head injury. J Neurosurg. 1991;7:545–51.
Diaz-Parejo P, Stahl N, Xu W, et al. Cerebral energy metabolism during transient hyperglycemia in patients with severe brain trauma. Intensive Care Med. 2003;29:544–50.
Liu-DeRyke X, Collingridge DS, Orme J, et al. Clinical impact of hyperglycemia during acute phase of traumatic brain injury. Neurocrit Care. 2009;11:151–7.
Van Den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. NEJM. 2001;345:1359–67.
Van den Berghe G, Wouters PJ, Bouillon R, et al. Outcome benefit of intensive insulin therapy in the critically ill: insulin dose versus glycemic control. Crit Care Med. 2003;31:359–66.
Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. NEJM. 2006;354:449–61.
The NICE-SUGAR Investigators. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–97.
Davidson P, Steed RD, Bode BW. A computer-directed intravenous insulin system shown to be safe, simple, and effective in 120, 618 h of operation. Diabetes Care. 2005;28:2418–23.
Vespa P, Boonyaputthikul R, McArthur DL, et al. Intensive insulin therapy reduces microdialysis glucose values without altering glucose utilization or improving the lactate/pyruvate ratio after traumatic brain injury. Crit Care Med. 2006;34(3):850–6.
Oddo M, Schmidt JM, Carrera E, et al. Impact of tight glycemic control on cerebral glucose metabolism after severe brain injury: a microdialysis study. Crit Care Med. 2008;36(12):3233–8.
Wiener RS, Wiener DC, Larson FJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA. 2008;300:933–44.
Acknowledgments
This research was supported by grants from the American Heart Association, HMSA Foundation, Abbott Laboratories, and The Queen Emma Foundation. The Glucommander software was provided by American Healthways for a several month trial period though not specifically for the study, and was later purchased by the medical center. The findings and conclusions of this study do not necessarily represent the views of The Queen’s Medical Center. Special thanks to the following people who contributed to the data collection, statistical analysis and advice regarding the study: Gheorghe Doros, PhD, Harlan Meyer, MD, PhD, Johnna Delcastillo, Lyle Oshita, RN, Edward Helekahi, RN, Eileen Cacal, RN, and Lisa Wagner, RN.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Green, D.M., O’Phelan, K.H., Bassin, S.L. et al. Intensive Versus Conventional Insulin Therapy in Critically Ill Neurologic Patients. Neurocrit Care 13, 299–306 (2010). https://doi.org/10.1007/s12028-010-9417-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-010-9417-3