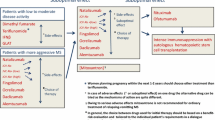
Abstract
Multiple sclerosis (MS) is a chronic inflammatory autoimmune disease of the central nervous system. It is characterized by demyelination of neurons and loss of neuronal axons and oligodendrocytes. In MS, auto-reactive T cells and B cells cross the blood–brain barrier (BBB), causing perivenous demyelinating lesions that form multiple discrete inflammatory demyelinated plaques located primarily in the white matter. In chronic MS, cortical demyelination and progressive axonal transections develop. Treatment for MS can be stratified into disease-modifying therapies (DMTs) and symptomatic therapy. DMTs aim to decrease circulating immune cells or to prevent these cells from crossing the BBB and reduce the inflammatory response. There are currently 10 DMTs approved for the relapsing forms of MS; these vary with regard to their efficacy, route and frequency of administration, adverse effects, and toxicity profile. Better drug delivery systems are being developed in order to decrease adverse effects, increase drug efficacy, and increase patient compliance through the direct targeting of pathologic cells. Here, we address the uses and benefits of advanced drug delivery systems, including nanoparticles, microparticles, fusion antibodies, and liposomal formulations. By altering the properties of therapeutic particles and enhancing targeting, breakthrough drug delivery technologies potentially applicable to multiple disease treatments may rapidly emerge.



Similar content being viewed by others
References
Wingerchuk DM, Carter JL. Multiple sclerosis: current and emerging disease-modifying therapies and treatment strategies. Mayo Clin Proc. 2014;89:225–40.
Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bö L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–85.
Alonso A, Hernán MA. Temporal trends in the incidence of multiple sclerosis: a systematic review. Neurology. 2008;71:129–35.
Files DK, Jausurawong T, Katrajian T, Danoff R. Multiple sclerosis. Prim Care Clin Off Pract. 2015;42:159–72.
Kamm CP, Uitdehaag BM, Polman CH. Multiple sclerosis: current knowledge and future outlook. Eur Neurol. 2014;72:132–41.
Prat E, Tomaru U, Sabater L, Park D, Granger R, Kruse N, Ohayon J, et al. HLA-DRB5*0101 and -DRB1*1501 expression in the multiple sclerosis-associated HLA-DR15 haplotype. J Neuroimmunol. 2005;167:108–19.
Kaushansky N, Altmann DM, Ascough S, David CS, Lassmann H, Ben-Nun A. HLA-DQB1*0602 determines disease susceptibility in a new “humanized” multiple sclerosis model in HLA-DR15 (DRB1*1501;DQB1*0602) transgenic mice. J Immunol. 2009;183:3531–41.
Calabresi PA. Diagnosis and management of multiple sclerosis. Am Fam Physician. 2004;70:1935–44.
Dhib-Jalbut S, Marks S. Interferon-β mechanisms of action in multiple sclerosis. Neurology. 2010;74:S17–24.
Khan O, Dhib-Jalbut S. Neutralizing antibodies to interferon β-1a and interferon β-1b in MS patients are cross-reactive. Neurology. 1998;51:1698–702.
Wingerchuk D. Multiple sclerosis disease-modifying therapies: adverse effect surveillance and management. Expert Rev Neurother. 2006;6:333–46.
Lalive PH, Neuhaus O, Benkhoucha M, Burger D, Hohlfeld R, Zamvil SS, Weber MS. Glatiramer acetate in the treatment of multiple sclerosis: emerging concepts regarding its mechanism of action. CNS Drugs. 2011;25:401–14.
Aharoni R, Arnon R. Enhanced myelination in autoimmunity and in normal development induced by glatiramer acetate. Isr Med Assoc J. 2014;16:611–3.
Arnon R, Sela M. Immunomodulation by the copolymer glatiramer acetate. J Mol Recognit. 2003;16:412–21.
Karussia D, Teitelnaum D, Sicsic C, Brenner T. Long-term treatment of multiple sclerosis with glatiramer acetate: natural history of the subtypes of anti-glatiramer acetate antibodies and their correlation with clinical efficacy. J Neuroimmunol. 2010;220:125–30.
Cohen J, Chun J. Mechanisms of fingolimod’s efficacy and adverse effects in multiple sclerosis. Ann Neurol. 2011;69:759–77.
Claussen M, Korn T. Immune mechanisms of new therapeutic strategies in MS: teriflunomide. Clin Immunol. 2012;142:49–56.
Bomprezzi R. Dimethyl fumarate in the treatment of relapsing–remitting multiple sclerosis: an overview. Ther Adv Neurol Disord. 2015;8:20–30.
Linker R, Gold R. Dimethyl fumarate for treatment of multiple sclerosis: mechanism of action, effectiveness, and side effects. Curr Neurol Neursci Rep. 2013;13:394.
Burton JM, O’Connor PW, Hohol M, Beyene J. Oral versus intravenous steroids for treatment of relapses in multiple sclerosis. Cochrane Database Syst Rev. 2009;3:CD006921.
Gaillard PJ, Appeldoorn CCM, Rip J, Dorland R, Van Der Pol SMA, Kooij G, Vries HE, Reijerkerk A. Enhanced brain delivery of liposomal methylprednisolone improved therapeutic efficacy in a model of neuroinflammation. J Control Release. 2012;164:364–9.
Ransohoff R. Natalizumab for multiple sclerosis. N Engl J Med. 2007;356:2622–9.
He D, Guo R, Zhang F, Zhang C, Dong S, Zhou H. Rituximab for relapsing–remitting multiple sclerosis. Cochrane Database Syst Rev. 2013;12:009130.
Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, Bar-Or A, Panzara M, Sarkar N, Agarwal S, Langer-Gould A, Smith CH. B-cell depletion with rituximab in relapsing–remitting multiple sclerosis. N Engl J Med. 2008;358:676–88.
Klein C, Lammens A, Schäfer W, Georges G, Schwaiger M, Mössner E, Hopfner KP, et al. Epitope interactions of monoclonal antibodies targeting CD20 and their relationship to functional properties. MAbs. 2013;5:22–33.
Jean GW, Comeau JM. Role of obinutuzumab in the treatment of chronic lymphocytic leukemia. Am J Health Syst Pharm. 2015;72:933–42.
English C, Aloi JJ. New FDA-approved disease-modifying therapies for multiple sclerosis. Clin Ther. 2015;37:691–715.
Cossburn M, Pace AA, Jones J, Ali R, Ingram G, Baker K, Hirst C, et al. Autoimmune disease after alemtuzumab treatment for multiple sclerosis in a multicenter cohort. Neurology. 2011;77:573–9.
Le Deley ML, Suzan F, Cutuli B, Delaloge S, Shamsaldin A, Linassier C, Clisant S, et al. Anthracyclines, mitoxantrone, radiotherapy, and granulocyte colony-stimulating factor: risk factors for leukemia and myelodysplastic syndrome after breast cancer. J Clin Oncol. 2007;25:292–300.
Scott LJ, Figgitt DP. Mitoxantrone: a review of its use in multiple sclerosis. CNS Drugs. 2004;18:379–96.
Todaro M, Zeuner A, Stassi G. Role of apoptosis in autoimmunity. J Clin Immunol. 2004;24:1–11.
Pender MP, Rist MJ. Apoptosis of inflammatory cells in immune control of the nervous system: role of glia. Glia. 2001;36:137–44.
Hebb ALO, Moore CS, Bhan V, Robertson GS. Targeting apoptosis to treat multiple sclerosis. Curr Drug Discov Technol. 2008;5:75–7.
Wosik K, Antel J, Kuhlmann T, Brück W, Massie B, Nalbantoglu J. Oligodendrocyte injury in multiple sclerosis: a role for p53. J Neurochem. 2003;85:635–44.
Sharief MK, Noori MA, Zoukos Y. Reduced expression of the inhibitor of apoptosis proteins in T cells from patients with multiple sclerosis following interferon-beta therapy. J Neuroimmunol. 2002;129:224–31.
Zehntner SP, Bourbonnière L, Moore CS, Morris SJ, Methot D, St Jean M, Lacasse E, et al. X-linked inhibitor of apoptosis regulates T cell effector function. J Immunol. 2007;179:7553–60.
Zhang Z, Tongchusak S, Mizukami Y, Kang YJ, Ioji T, Touma M, Reinhold B, et al. Induction of anti-tumor cytotoxic T cell responses through PLGA-nanoparticle mediated antigen delivery. Biomaterials. 2011;32:3666–78.
Lewis JS, Zaveri TD, Crooks CP, Keselowsky BG. Microparticle surface modifications targeting dendritic cells for non-activating applications. Biomaterials. 2012;33:7221–32.
Lu CH, Willner B, Willner I. DNA nanotechnology: from sensing and DNA machines to drug-delivery systems. ACS Nano. 2013;7:8320–32.
Getts DR, Martin AJ, McCarthy DP, Terry RL, Hunter ZN, Yap WT, Getts MT, et al. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat Biotechnol. 2012;30:1217–24.
Tsai M, Lu Z, Wientjes MG, Au JLS. Paclitaxel-loaded polymeric microparticles: quantitative relationships between in vitro drug release rate and in vivo pharmacodynamics. J Control Release. 2013;172:737–44.
Youan B, Benoit M, Baras B, Gillard J. Protein-loaded poly(epsilon-caprolactone) microparticles. I. Optimization of the preparation by (water-in-oil)-in water emulsion solvent evaporation. J Microencapsul. 1999;16:587–99.
Jones SA, Martin GP, Brown MB. Manipulation of beclomethasone–hydrofluoroalkane interactions using biocompatible macromolecules. J Pharm Sci. 2006;95:1060–74.
Grizzi I, Garreau H, Li S, Vert M. Hydrolytic degradation of devices based on poly(DL-lactic acid) size dependence. Biomaterials. 1995;16:305–11.
Kim SE, Park JH, Cho YW, Chung H, Jeong SY, Lee EB, Kwon IC. Porous chitosan scaffold containing microspheres loaded with transforming growth factor-β1: implications for cartilage tissue engineering. J Control Release. 2003;91:365–74.
Holland TA, Tabata Y, Mikos AG. In vitro release of transforming growth factor-β1 from gelatin microparticles encapsulated in biodegradable, injectable oligo(poly(ethylene glycol) fumarate) hydrogels. J Control Release. 2003;91:299–313.
Berthold A, Cremer K, Kreuter J. Collagen microparticles: carriers for glucocorticosteroids. Eur J Pharm Biopharm. 1998;45:23–9.
Bowersock TL, HogenEsch H, Suckow M, Porter RE, Jackson R, Park H, Park K. Oral vaccination with alginate microsphere systems. J Control Release. 1996;39:209–20.
Yuan L, Tang Q, Yang D, Zhang JZ, Zhang F, Hu J. Preparation of pH-responsive mesoporous silica nanoparticles and their application in controlled drug delivery. J Phys Chem C. 2011;115:9926–32.
Wei G, Pettway GJ, McCauley LK, Ma PX. The release profiles and bioactivity of parathyroid hormone from poly (lactic-co-glycolic acid) microspheres. Biomaterials. 2004;25:345–52.
Wischke C, Schwendeman S. Principles of encapsulating hydrophobic drugs in PLA/PLGA microparticles. Int J Pharm. 2008;364:298–327.
Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat Med. 2010;16:1035–41.
Hubbell JA, Thomas SN, Swartz MA. Materials engineering for immunomodulation. Nature. 2009;462:449–60.
Ihler GM, Glew RH, Schnure FW. Enzyme loading of erythrocytes. Proc Natl Acad Sci USA. 1973;70:2663–6.
Steinman RM, Gutchinov B, Witmer MD, Nussenzweig MC. Dendritic cells are the principal stimulators of the primary mixed leukocyte reaction in mice. J Exp Med. 1983;157:613–27.
Petzold C, Schallenberg S, Stern JNH, Kretschmer K. Targeted antigen delivery to DEC-205+ dendritic cells for tolerogenic vaccination. Rev Diabet Stud. 2012;9:305–18.
Stern JNH, Keskin DB, Kato Z, Waldner H, Schallenberg S, Anderson A, von Boehmer H, et al. Promoting tolerance to proteolipid protein-induced experimental autoimmune encephalomyelitis through targeting dendritic cells. Proc Natl Acad Sci USA. 2010;107:17280–5.
Serafini B, Columba-Cabezas S, Di Risa F, Aloisi F. Intracerebral recruitment and maturation of dendritic cells in the onset and progression of experimental autoimmune encephalomyelitis. Am J Pathol. 2000;157:1991–2002.
Izikson L. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR) 2. J Exp Med. 2000;192:1075–80.
Greter M, Heppner F, Lemos M, Odermatt B, Goebels N, Laufer T, Noelle R, et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med. 2005;11:328–34.
McMenamin P. Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations. J Comp Neurol. 1999;405:553–62.
Bailey S, Schreiner B, McMahon E, Miller S. CNS myeloid DCs presenting endogenous myelin peptides “preferentially” polarize CD4+ T(H)-17 cells in relapsing EAE. Nat Immunol. 2007;8:172–80.
Lande R. Plasmacytoid dendritic cells in multiple sclerosis: intracerebral recruitment and impaired maturation in response to interferon-beta. J Neuropathol Exp Neurol. 2008;67:388–401.
Gottfried-Blackmore A, Krauzner U, Idoyaga J, Felger J, McEwen B, Bulloch K. Acute in vivo exposure to interferon-gamma enables resident brain dendritic cells to become effective antigen presenting cells. Proc Natl Acad Sci USA. 2009;106:20918–23.
Cao Y, Goods B, Raddassi K, Nepom G, Kwok W, Love J, Hafler D. Functional inflammatory profiles distinguish myelin-reactive T cells from patients with multiple sclerosis. Sci Transl Med. 2015;7:287ra74.
Bettelli E, Sullivan B, Szabo SJ, Sobel RA, Glimcher LH, Kuchroo VK. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J Exp Med. 2004;200:79–87.
Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517.
Ghoreschi K, Laurence A, Yang X-P, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-β signalling. Nature. 2010;467:967–71.
Janeway C, Travers J, Walport M, Shlomchik M. The production of armed effector T cells. In: Immunobiology: the immune system in health and disease. 5th ed. New York: Garland Science; 2001. ISBN-10: 0-8153-3642-X.
Kool M, Soullié T, van Nimwegen M, Willart MAM, Muskens F, Jung S, Hoogsteden HC, et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205:869–82.
Sharp FA, Ruane D, Claass B, Creagh E, Harris J, Malyala P, Singh M, et al. Uptake of particulate vaccine adjuvants by dendritic cells activates the NALP3 inflammasome. Proc Natl Acad Sci USA. 2009;106:870–5.
Kato M, McDonald KJ, Khan S, Ross IL, Vuckovic S, Chen K, Munster D, et al. Expression of human DEC-205 (CD205) multilectin receptor on leukocytes. Int Immunol. 2006;18:857–69.
Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–38.
Champion JA, Walker A, Mitragotri S. Role of particle size in phagocytosis of polymeric microspheres. Pharm Res. 2008;25:1815–21.
Tran K, Shen H. The role of phagosomal pH on the size-dependent efficiency of cross-presentation by dendritic cells. Biomaterials. 2009;30:1356–62.
Reddy ST, van der Vlies AJ, Simeoni E, O’Neil CP, Swartz MA, Hubbell JA. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25:1159–64.
Reddy ST, Rehor A, Schmoekel HG, Hubbell JA, Swartz MA. In vivo targeting of dendritic cells in lymph nodes with poly (propylene sulfide) nanoparticles. J Control Release. 2006;112:26–34.
Idoyaga J, Fiorese C, Zbytnuik L, Lubkin A, Miller J, Malissen B, Mucida D, et al. Specialized role of migratory dendritic cells in peripheral tolerance induction. J Clin Invest. 2013;123:844–54.
Jones A, Opejin A, Henderson JG, Gross C, Jain R, Epstein JA, Flavell RA, et al. Peripherally induced tolerance depends on peripheral regulatory T cells that require Hopx to inhibit intrinsic IL-2 expression. J Immunol. 2015;195:1489–97.
Hawiger D, Masilamani RF, Bettelli E, Kuchroo VK, Nussenzweig MC. Immunological unresponsiveness characterized by increased expression of CD5 on peripheral T cells induced by dendritic cells in vivo. Immunity. 2004;20:695–705.
Nussenzweig MC, Steinman RM, Witmer MD, Gutchinov B. A monoclonal antibody specific for mouse dendritic cells. Proc Natl Acad Sci USA. 1982;79:161–5.
Kasahara S, Clark EA. Dendritic cell-associated lectin 2 (DCAL2) defines a distinct CD8α- dendritic cell subset. J Leukoc Biol. 2012;2012(91):437–48.
Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–11.
Nagae M, Yamanaka K, Hanashima S, Ikeda A, Morita-Matsumoto K, Satoh T, Matsumoto N, et al. Recognition of bisecting N-acetylglucosamine: structural basis for asymmetric interaction with the mouse lectin dendritic cell inhibitory receptor 2. J Biol Chem. 2013;288:33598–610.
Yamazaki S, Dudziak D, Heidkamp GF, Fiorese C, Bonito AJ, Inaba K, Nussenzweig MC, et al. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J Immunol. 2008;181:6923–33.
Yamazaki S, Dudziak D, Heidkamp GF, Fiorese C, Bonito AJ, Inaba K, Nussenzweig MC, et al. DCIR2+ cDC2 DCs and Zbtb32 restore CD4+ T cell tolerance and inhibit diabetes. Diabetes. 2015;64:3521–31.
Hunter Z, McCarthy D, Yap W, Harp C, Getts D, Shea L, Miller S. A biodegradable nanoparticle platform for the induction of antigen-specific immune tolerance for treatment of autoimmune disease. ACS Nano. 2014;8:2148–60.
White M, Webster G, O’Sullivan D, Stone S, La Flamme AC. Targeting innate receptors with MIS416 reshapes Th responses and suppresses CNS disease in a mouse model of multiple sclerosis. PLoS One. 2014;9:e87712.
Maldonado R, LaMothe R, Ferrari J, Rossi R, Kolte P, Griset A, O’Neil C, et al. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc Natl Acad Sci USA. 2015;112:156–65.
Liu N, Dunphy DR, Atanassov P, Bunge SD, Chen Z, López GP, Boyle TJ, et al. Photoregulation of mass transport through a photoresponsive azobenzene-modified nanoporous membrane. Nano Lett. 2004;4:551–4.
Khashab N, Trablosi A, Lau Y, Ambrogio M, Friedman D, Khatib H, Zink J, et al. Redox- and pH-controlled mechanized nanoparticles. Eur J Org Chem. 2009;2009(11):1669–73.
Zhao Y, Trewyn BG, Slowing II, Lin VSY. Mesoporous silica nanoparticle-based double drug delivery system for glucose-responsive controlled release of insulin and cyclic AMP. J Am Chem Soc. 2009;131:8398–400.
Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–34.
Almqvist KF, Dhollander A, Verdonk P, Forsyth R, Verdonk R, Verbruggen G. Treatment of cartilage defects in the knee using alginate beads containing human mature allogenic chondrocytes. Am J Sports Med. 2009;37:1920–9.
Gordon AN, Fleagle JT, Guthrie D, Parkin DE, Gore ME, Lacave AJ. Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol. 2001;2002(19):3312–22.
Alberts DS, Muggia FM, Carmichael J, Winer EP, Jahanzeb M, Venook AP, Skubitz KM, Rivera E, Sparano JA, Dibella NJ, Stewart SJ, Kavanagh JJ, Gabizon AA. Efficacy and safety of liposomal anthracyclines in phase I/II clinical trials. Semin Oncol. 2004;2001(31):53–90.
Koukourakis MI, Koukouraki S, Fezoulidis I, Kelekis N, Kyrias G, Archimandritis S, Karkavitsas N. High intratumoural accumulation of stealth liposomal doxorubicin (Caelyx®) in glioblastomas and in metastatic brain tumours. Br J Cancer. 2000;83:1281–6.
Harris L, Batist G, Belt R, Rovira D, Navari R, Azarnia N, Welles L, et al. Liposome-encapsulated doxorubicin compared with conventional doxorubicin in a randomized multicenter trial as first-line therapy of metastatic breast carcinoma. Cancer. 2002;94:25–36.
Batist G, Barton J, Chaikin P, Swenson C, Welles L. Myocet (liposome-encapsulated doxorubicin citrate): a new approach in breast cancer therapy. Expert Opin Pharmacother. 2002;3:1739–51.
Inaba K, Swiggard W, Inaba M, Meltzer J, Mirza A, Sasagawa T, Nussenzweig MC, et al. Tissue distribution of the DEC-205 protein that is detected by the monoclonal antibody NLDC-145. I. Expression on dendritic cells and other subsets of mouse leukocytes. Cell Immunol. 1995;163:148–56.
Guo M, Gong S, Maric S, Misulovin Z, Pack M, Mahnke K, Nussenzweig MC, et al. A monoclonal antibody to the DEC-205 endocytosis receptor on human dendritic cells. Hum Immunol. 2000;61:729–38.
Acknowledgments
We are very grateful to Leah Messina of Approach Positive Marketing for designing Figs. 1, 2, and 3 within this review. I.T. was funded by the Bristol Myers Squibb Postdoctoral Fellowship and by NIH F32 HD081835. M.M was supported by a fellowship from the Cheryl Manne Fund for the Cure of MS. J.N.H.S was funded by a grant from the HT Langbert Charitable Trust Research Fund. P.W., K.B.S., M.M., and J.N.H.S. are associates of the Northeast NMO Consortium. We also thank Marlena Kern and Gila Klein for their assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Additional information
Inna Tabansky, Mark D. Messina and Joel N. H. Stern have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Tabansky, I., Messina, M.D., Bangeranye, C. et al. Advancing drug delivery systems for the treatment of multiple sclerosis. Immunol Res 63, 58–69 (2015). https://doi.org/10.1007/s12026-015-8719-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-015-8719-0