Abstract
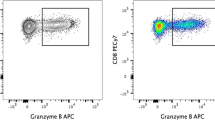
Flow cytometry-based analysis of T-cell receptor (TCR) repertoires is an essential tool for the detection of clonal T-cell expansions in physiologic and pathologic conditions. Individual T-cell subsets can be investigated based on their surface properties. The aims of our study were to provide reference values for various disease settings and delineate the contribution of individual TCR repertoires to the human T-cell differentiation pathway. We analyzed blood of 66 healthy subjects aged 0 (cord blood) to 72 years. Lymphocyte subpopulations and TCR repertoires were simultaneously explored using antibodies specific to CD3, CD4, CD8, CD45RA, CCR7, CD27, CD57 and a set of 25 antibodies detecting human TCR-Vβ chains. Statistical analysis included Wilcoxon, paired t and ANOVA tests. Initially, TCR expansion values were calculated based on the analysis of TCR-Vβ distribution on CD4+ and CD8+ T cells. We then established gating strategies and an algorithm for data analysis allowing for discrimination of T-cell subsets and TCR distribution. Dominant TCR expansions were present within effector as opposed to central/effector memory or naive cells, e.g., median TCR-Vβ expansion rate was highest on CD45RA+/CCR7− effector CD4+/8+ cells (eight and 11-fold, respectively). Remarkably, TCR expansions were missing (0) or very low (0.5) on CD4+ and CD8+ central memory population, respectively. No significant gender-related variability of TCR repertoires was identified, and significant impact of chronic cytomegalovirus infection was shown. Our results serve as reference for future studies elucidating clonal TCR dominance of T-cell subsets.





Similar content being viewed by others
References
Bronke C, Westerlaken GH, Miedema F, Tesselaar K, van Baarle D. Progression to cmv end-organ disease in hiv-1-infected individuals despite abundance of highly differentiated cmv-specific cd8 + t-cells. Immunol Lett. 2005;97:215–24.
Fritsch RD, Shen X, Sims GP, Hathcock KS, Hodes RJ, Lipsky PE. Stepwise differentiation of cd4 memory t cells defined by expression of ccr7 and cd27. J Immunol. 2005;175:6489–97.
Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory t cell subsets: function, generation, and maintenance. Ann Rev Immunol. 2004;22:745–63.
van Baarle D, Kostense S, van Oers MH, Hamann D, Miedema F. Failing immune control as a result of impaired cd8 + t-cell maturation: Cd27 might provide a clue. Trends Immunol. 2002;23:586–91.
Lefranc MP, Rabbitts TH. Two tandemly organized human genes encoding the t-cell gamma constant-region sequences show multiple rearrangement in different t-cell types. Nature. 1985;316:464–6.
Meier JT, Lewis SM. P nucleotides in v(d)j recombination: a fine-structure analysis. Mol Cellular Biol. 1993;13:1078–92.
Padovan E, Casorati G, Dellabona P, Meyer S, Brockhaus M, Lanzavecchia A. Expression of two t cell receptor alpha chains: dual receptor t cells. Science. 1993;262:422–4.
Clemente MJ, Wlodarski MW, Makishima H, Viny AD, Bretschneider I, Shaik M, Lichtin AE, Hsi ED, et al. Clonal drift demonstrates unexpected dynamics of the t-cell repertoire in t-large granular lymphocyte leukemia. Blood. 2011;118:4384–93.
Fozza C, Longinotti M. T-cell receptor repertoire usage in hematologic malignancies. Critical reviews in oncology/hematology 2012.
Luo W, Su J, Zhang XB, Yang Z, Zhou MQ, Jiang ZM, Hao PP, Liu SD, et al. Limited t cell receptor repertoire diversity in tuberculosis patients correlates with clinical severity. PLoS ONE. 2012;7:e48117.
Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–58.
Wei S, Charmley P, Robinson MA, Concannon P. The extent of the human germline t-cell receptor v beta gene segment repertoire. Immunogenetics. 1994;40:27–36.
McLean-Tooke A, Barge D, Spickett GP, Gennery AR. T cell receptor vbeta repertoire of t lymphocytes and t regulatory cells by flow cytometric analysis in healthy children. Clin Exp Immunol. 2008;151:190–8.
Bonfigli S, Doro MG, Fozza C, Derudas D, Dore F, Longinotti M. T-cell receptor repertoire in healthy sardinian subjects. Hum Immunol. 2003;64:689–95.
MacIsaac C, Curtis N, Cade J, Visvanathan K. Rapid analysis of the vbeta repertoire of cd4 and cd8 t lymphocytes in whole blood. J Immunol Methods. 2003;283:9–15.
van den Beemd R, Boor PP, van Lochem EG, Hop WC, Langerak AW, Wolvers-Tettero IL, Hooijkaas H, van Dongen JJ. Flow cytometric analysis of the Vβ repertoire in healthy controls. Cytometry. 2000;40:336–45.
Degauque N, Boeffard F, Foucher Y, Ballet C, Brouard S, Soulillou JP. The blood of healthy individuals exhibits cd8 t cells with a highly altered tcr vb repertoire but with an unmodified phenotype. PLoS ONE. 2011;6:e21240.
Klarenbeek PL, Tak PP, van Schaik BD, Zwinderman AH, Jakobs ME, Zhang Z, van Kamper AH, van Lier RA, et al. Human t-cell memory consists mainly of unexpanded clones. Immunol Lett. 2010;133:42–8.
Shendure J, Ji H. Next-generation DNA sequencing. Nat Biotechnol. 2008;26:1135–45.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bretschneider, I., Clemente, M.J., Meisel, C. et al. Discrimination of T-cell subsets and T-cell receptor repertoire distribution. Immunol Res 58, 20–27 (2014). https://doi.org/10.1007/s12026-013-8473-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-013-8473-0