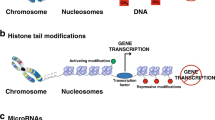
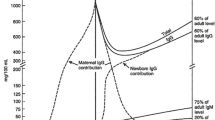
Abstract
Neonatal animals have heightened susceptibility to infectious agents and are at increased risk for the development of allergic diseases, such as asthma. Experimental studies using animal models have been quite useful for beginning to identify the cellular and molecular mechanisms underlying these sensitivities. In particular, results from murine neonatal models indicate that developmental regulation of multiple immune cell types contributes to the typically poor responses of neonates to pathogenic microorganisms. Surprisingly, however, animal studies have also revealed that responses at mucosal surfaces in early life may be protective against primary or secondary disease. Our understanding of the molecular events underlying these processes is less well developed. Emerging evidence indicates that the functional properties of neonatal immune cells and the subsequent maturation of the immune system in ontogeny may be regulated by epigenetic phenomena. Here, we review recent findings from our group and others describing cellular responses to infection and developmentally regulated epigenetic processes in the newborn.
Similar content being viewed by others
References
Rowe J, Macaubas C, Monger T, Holt BJ, Harvey J, Poolman JT, et al. Heterogeneity in diphtheria-tetanus-acellular pertussis vaccine-specific cellular immunity during infancy: relationship to variations in the kinetics of postnatal maturation of systemic th1 function. J Infect Dis. 2001;184(1):80–8. doi:10.1086/320996.
Webster RB, Rodriguez Y, Klimecki WT, Vercelli D. The human IL-13 locus in neonatal CD4+ T cells is refractory to the acquisition of a repressive chromatin architecture. J Biol Chem. 2007;282(1):700–9.
Ribeiro-do-Couto LM, Boeije LC, Kroon JS, Hooibrink B, Breur-Vriesendorp BS, Aarden LA, et al. High IL-13 production by human neonatal T cells: neonate immune system regulator? Eur J Immunol. 2001;31(11):3394–402.
Schaub B, Liu J, Schleich I, Hoppler S, Sattler C, von Mutius E. Impairment of T helper and T regulatory cell responses at birth. Allergy. 2008;63(11):1438–47.
Prescott SL, Macaubas C, Holt BJ, Smallacombe TB, Loh R, Sly PD, et al. Transplacental priming of the human immune system to environmental allergens: universal skewing of initial T cell responses toward the Th2 cytokine profile. J Immunol. 1998;160(10):4730–7.
Gans H, Yasukawa L, Rinki M, DeHovitz R, Forghani B, Beeler J, et al. Immune responses to measles and mumps vaccination of infants at 6, 9, and 12 months. J Infect Dis. 2001;184(7):817–26.
Gans HA, Maldonado Y, Yasukawa LL, Beeler J, Audet S, Rinki MM, et al. IL-12, IFN-gamma, and T cell proliferation to measles in immunized infants. J Immunol. 1999;162(9):5569–75.
Gans HA, Arvin AM, Galinus J, Logan L, DeHovitz R, Maldonado Y. Deficiency of the humoral immune response to measles vaccine in infants immunized at age 6 months. JAMA. 1998;280(6):527–32. doi:10.1001/jama.280.6.527.
Gans H, DeHovitz R, Forghani B, Beeler J, Maldonado Y, Arvin AM. Measles and mumps vaccination as a model to investigate the developing immune system: passive and active immunity during the first year of life. Vaccine. 2003;21(24):3398–405. doi:10.1016/S0264-410X(03)00341-4.
White OJ, Rowe J, Richmond P, Marshall H, McIntyre P, Wood N, et al. Th2-polarisation of cellular immune memory to neonatal pertussis vaccination. Vaccine. 2010;28(14):2648–52. doi:10.1016/j.vaccine.2010.01.010.
Mascart F, Hainaut M, Peltier A, Verscheure V, Levy J, Locht C. Modulation of the infant immune responses by the first pertussis vaccine administrations. Vaccine. 2007;25(2):391–8. doi:10.1016/j.vaccine.2006.06.046.
Renz H, Brandtzaeg P, Hornef M. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nat Rev Immunol. 2011;12(1):9–23. doi:10.1038/nri3112.
Cuenca AG, Wynn JL, Moldawer LL, Levy O. Role of innate immunity in neonatal infection. Am J Perinatol. 2013;30(2):105–12. doi:10.1055/s-0032-1333412.
Guilmot A, Hermann E, Braud VM, Carlier Y, Truyens C. Natural killer cell responses to infections in early life. J Innate Immun. 2011;3(3):280–8. doi:10.1159/000323934.
Ygberg S, Nilsson A. The developing immune system—from foetus to toddler. Acta Paediatr. 2011;101(2):120–7. doi:10.1111/j.1651-2227.2011.02494.x.
Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol. 2009;9(3):185–94. doi:10.1038/nri2508.
Willems F, Vollstedt S, Suter M. Phenotype and function of neonatal DC. Eur J Immunol. 2009;39(1):26–35.
Mold JE, McCune JM. Immunological tolerance during fetal development: from mouse to man. Adv Immunol. 2012;115:73–111. doi:10.1016/B978-0-12-394299-9.00003-5.
Kollmann TR, Levy O, Montgomery RR, Goriely S. Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity. 2012;37(5):771–83. doi:10.1016/j.immuni.2012.10.014.
Vekemans J, Amedei A, Ota MO, D’Elios MM, Goetghebuer T, Ismaili J, et al. Neonatal bacillus Calmette-Guerin vaccination induces adult-like IFN-gamma production by CD4+ T lymphocytes. Eur J Immunol. 2001;31(5):1531–5.
Ryan M, Murphy G, Ryan E, Nilsson L, Shackley F, Gothefors L, et al. Distinct T-cell subtypes induced with whole cell and acellular pertussis vaccines in children. Immunology. 1998;93(1):1–10.
Ausiello CM, Urbani F, la Sala A, Lande R, Cassone A. Vaccine- and antigen-dependent type 1 and type 2 cytokine induction after primary vaccination of infants with whole-cell or acellular pertussis. Infect Immun. 1997;65:2168–74.
Black A, Bhaumik S, Kirkman RL, Weaver CT, Randolph DA. Developmental regulation of Th17-cell capacity in human neonates. Eur J Immunol. 2012;42(2):311–9. doi:10.1002/eji.201141847.
Cosmi L, De Palma R, Santarlasci V, Maggi L, Capone M, Frosali F, et al. Human interleukin 17-producing cells originate from a CD161+ CD4+ T cell precursor. J Exp Med. 2008;205(8):1903–16. doi:10.1084/jem.20080397.
Echeverry A, Schesser K, Adkins B. Murine neonates are highly resistant to Yersinia enterocolitica following orogastric exposure. Infect Immun. 2007;75(5):2234–43.
Echeverry A, Saijo S, Schesser K, Adkins B. Yersinia enterocolitica promotes robust mucosal inflammatory T-cell immunity in murine neonates. Infect Immun. 2010;78(8):3595–608.
Glode MP, Sutton A, Moxon ER, Robbins JB. Pathogenesis of neonatal Escherichia coli meningitis: induction of bacteremia and meningitis in infant rats fed E. coli K1. Infect Immun. 1977;16(1):75–80.
Pluschke G, Mercer A, Kusecek B, Pohl A, Achtman M. Induction of bacteremia in newborn rats by Escherichia coli K1 is correlated with only certain O (lipopolysaccharide) antigen types. Infect Immun. 1983;39(2):599–608.
Mushtaq N, Redpath MB, Luzio JP, Taylor PW. Prevention and cure of systemic Escherichia coli K1 infection by modification of the bacterial phenotype. Antimicrob Agents Chemother. 2004;48(5):1503–8.
Mushtaq N, Redpath MB, Luzio JP, Taylor PW. Treatment of experimental Escherichia coli infection with recombinant bacteriophage-derived capsule depolymerase. J antimicrob chemother. 2005;56(1):160–5. doi:10.1093/jac/dki177.
Martindale J, Stroud D, Moxon ER, Tang CM. Genetic analysis of Escherichia coli K1 gastrointestinal colonization. Mol Microbiol. 2000;37(6):1293–305.
Zelmer A, Bowen M, Jokilammi A, Finne J, Luzio JP, Taylor PW. Differential expression of the polysialyl capsule during blood-to-brain transit of neuropathogenic Escherichia coli K1. Microbiology. 2008;154(Pt 8):2522–32. doi:10.1099/mic.0.2008/017988-0.
Zelmer A, Martin MJ, Gundogdu O, Birchenough G, Lever R, Wren BW, et al. Administration of capsule-selective endosialidase E minimizes upregulation of organ gene expression induced by experimental systemic infection with Escherichia coli K1. Microbiology. 2010;156(Pt 7):2205–15. doi:10.1099/mic.0.036145-0.
Birchenough GM, Johansson ME, Stabler RA, Dalgakiran F, Hansson GC, Wren BW, et al. Altered innate defenses in the neonatal gastrointestinal tract in response to colonization by neuropathogenic Escherichia coli. Infect Immun. 2013;81(9):3264–75. doi:10.1128/IAI.00268-13.
Fernandez MI, Thuizat A, Pedron T, Neutra M, Phalipon A, Sansonetti PJ. A newborn mouse model for the study of intestinal pathogenesis of shigellosis. Cell Microbiol. 2003;5(7):481–91.
Fernandez MI, Regnault B, Mulet C, Tanguy M, Jay P, Sansonetti PJ, et al. Maturation of paneth cells induces the refractory state of newborn mice to Shigella infection. J Immunol. 2008;180(7):4924–30.
Shim DH, Ryu S, Kweon MN. Defensins play a crucial role in protecting mice against oral Shigella flexneri infection. Biochem Biophys Res Commun. 2010;401(4):554–60. doi:10.1016/j.bbrc.2010.09.100.
Bry L, Falk P, Huttner K, Ouellette A, Midtvedt T, Gordon JI. Paneth cell differentiation in the developing intestine of normal and transgenic mice. Proc Natl Acad Sci USA. 1994;91(22):10335–9.
Ganz T, Sherman MP, Selsted ME, Lehrer RI. Newborn rabbit alveolar macrophages are deficient in two microbicidal cationic peptides, MCP-1 and MCP-2. Am Rev Respir Dis. 1985;132(4):901–4.
Ouellette AJ, Greco RM, James M, Frederick D, Naftilan J, Fallon JT. Developmental regulation of cryptdin, a corticostatin/defensin precursor mRNA in mouse small intestinal crypt epithelium. J Cell Biol. 1989;108(5):1687–95.
Huttner KM, Brezinski-Caliguri DJ, Mahoney MM, Diamond G. Antimicrobial peptide expression is developmentally regulated in the ovine gastrointestinal tract. J Nutr. 1998;128(2 Suppl):297S–9S.
Mallow EB, Harris A, Salzman N, Russell JP, DeBerardinis RJ, Ruchelli E, et al. Human enteric defensins. Gene structure and developmental expression. J Biol Chem. 1996;271(8):4038–45.
Starner TD, Agerberth B, Gudmundsson GH, McCray PB Jr. Expression and activity of beta-defensins and LL-37 in the developing human lung. J Immunol. 2005;174(3):1608–15.
Burger-van Paassen N, Loonen LM, Witte-Bouma J, Korteland-van Male AM, de Bruijn AC, van der Sluis M, et al. Mucin Muc2 deficiency and weaning influences the expression of the innate defense genes Reg3beta, Reg3gamma and angiogenin-4. PLoS ONE. 2012;7(6):e38798. doi:10.1371/journal.pone.0038798.
Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313(5790):1126–30. doi:10.1126/science.1127119.
Levast B, Schulz S, Hurk S, Gerdts V. Animal models for neonatal diseases in humans. Vaccine. 2013;31(21):2489–99. doi:10.1016/j.vaccine.2012.11.089.
Pott J, Stockinger S, Torow N, Smoczek A, Lindner C, McInerney G, et al. Age-dependent TLR3 expression of the intestinal epithelium contributes to rotavirus susceptibility. PLoS Pathog. 2012;8(5):e1002670. doi:10.1371/journal.ppat.1002670.
Lopez-Guerrero DV, Meza-Perez S, Ramirez-Pliego O, Santana-Calderon MA, Espino-Solis P, Gutierrez-Xicotencatl L, et al. Rotavirus infection activates dendritic cells from Peyer’s patches in adult mice. J Virol. 2010;84(4):1856–66. doi:10.1128/JVI.02640-08.
Weitkamp JH, Kallewaard N, Kusuhara K, Bures E, Williams JV, LaFleur B, et al. Infant and adult human B cell responses to rotavirus share common immunodominant variable gene repertoires. J Immunol. 2003;171(9):4680–8.
Blutt SE, Warfield KL, Lewis DE, Conner ME. Early response to rotavirus infection involves massive B cell activation. J Immunol. 2002;168(11):5716–21.
Rollo EE, Kumar KP, Reich NC, Cohen J, Angel J, Greenberg HB, et al. The epithelial cell response to rotavirus infection. J Immunol. 1999;163(8):4442–52.
Ramig RF. The effects of host age, virus dose, and virus strain on heterologous rotavirus infection of suckling mice. Microb Pathog. 1988;4(3):189–202. doi:10.1016/0882-4010(88)90069-1.
VanCott JL, Prada AE, McNeal MM, Stone SC, Basu M, Huffer B Jr, et al. Mice develop effective but delayed protective immune responses when immunized as neonates either intranasally with nonliving VP6/LT(R192G) or orally with live rhesus rotavirus vaccine candidates. J Virol. 2006;80(10):4949–61.
Arnold IC, Dehzad N, Reuter S, Martin H, Becher B, Taube C, et al. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J Clin Invest. 2011;121(8):3088–93. doi:10.1172/JCI45041.
Arnold IC, Lee JY, Amieva MR, Roers A, Flavell RA, Sparwasser T, et al. Tolerance rather than immunity protects from Helicobacter pylori-induced gastric preneoplasia. Gastroenterology. 2011;140(1):199–209. doi:10.1053/j.gastro.2010.06.047.
Oertli M, Sundquist M, Hitzler I, Engler DB, Arnold IC, Reuter S, et al. DC-derived IL-18 drives Treg differentiation, murine Helicobacter pylori-specific immune tolerance, and asthma protection. J Clin Invest. 2012;122(3):1082–96. doi:10.1172/JCI61029.
Burns-Guydish SM, Olomu IN, Zhao H, Wong RJ, Stevenson DK, Contag CH. Monitoring age-related susceptibility of young mice to oral Salmonella enterica serovar Typhimurium infection using an in vivo murine model. Pediatr Res. 2005;58(1):153–8.
Rhee SJ, Walker WA, Cherayil BJ. Developmentally regulated intestinal expression of IFN-gamma and its target genes and the age-specific response to enteric Salmonella infection. J Immunol. 2005;175(2):1127–36.
Garvy BA, Qureshi MH. Delayed inflammatory response to Pneumocystis carinii infection in neonatal mice is due to an inadequate lung environment. J Immunol. 2000;165(11):6480–6.
Empey KM, Hollifield M, Garvy BA. Exogenous heat-killed Escherichia coli improves alveolar macrophage activity and reduces Pneumocystis carinii lung burden in infant mice. Infect Immun. 2007;75(7):3382–93. doi:10.1128/IAI.00174-07.
Garvy BA, Harmsen AG. Susceptibility to Pneumocystis carinii infection: host responses of neonatal mice from immune or naive mothers and of immune or naive adults. Infect Immun. 1996;64(10):3987–92.
Qureshi MH, Garvy BA. Neonatal T cells in an adult lung environment are competent to resolve Pneumocystis carinii pneumonia. J Immunol. 2001;166(9):5704–11.
Qureshi MH, Cook-Mills J, Doherty DE, Garvy BA. TNF-alpha-dependent ICAM-1- and VCAM-1-mediated inflammatory responses are delayed in neonatal mice infected with Pneumocystis carinii. J Immunol. 2003;171(9):4700–7.
Qureshi MH, Empey KM, Garvy BA. Modulation of proinflammatory responses to Pneumocystis carinii f. sp. muris in neonatal mice by granulocyte-macrophage colony-stimulating factor and IL-4: role of APCs. J Immunol. 2005;174(1):441–8.
Kurkjian C, Hollifield M, Lines JL, Rogosky A, Empey KM, Qureshi M, et al. Alveolar macrophages in neonatal mice are inherently unresponsive to Pneumocystis murina infection. Infect Immun. 2012;80(8):2835–46. doi:10.1128/IAI.05707-11.
Lines JL, Hoskins S, Hollifield M, Cauley LS, Garvy BA. The migration of T cells in response to influenza virus is altered in neonatal mice. J Immunol. 2010;185(5):2980–8. doi:10.4049/jimmunol.0903075.
Tregoning JS, Yamaguchi Y, Harker J, Wang B, Openshaw PJ. The role of T cells in the enhancement of respiratory syncytial virus infection severity during adult reinfection of neonatally sensitized mice. J Virol. 2008;82(8):4115–24. doi:10.1128/JVI.02313-07.
Bhattacharya S, Beal BT, Janowski AM, Shornick LP. Reduced inflammation and altered innate response in neonates during paramyxoviral infection. Virol J. 2011;8:549. doi:10.1186/1743-422X-8-549.
Bonville CA, Ptaschinski C, Percopo CM, Rosenberg HF, Domachowske JB. Inflammatory responses to acute pneumovirus infection in neonatal mice. Virol J. 2010;7:320. doi:10.1186/1743-422X-7-320.
Dakhama A, Park JW, Taube C, Joetham A, Balhorn A, Miyahara N, et al. The enhancement or prevention of airway hyper responsiveness during reinfection with respiratory syncytial virus is critically dependent on the age at first infection and IL-13 production. J Immunol. 2005;175(3):1876–83.
Culley FJ, Pollott J, Openshaw PJ. Age at first viral infection determines the pattern of T cell-mediated disease during reinfection in adulthood. J Exp Med. 2002;196(10):1381–6.
Empey KM, Orend JG, Peebles RS Jr, Egana L, Norris KA, Oury TD, et al. Stimulation of immature lung macrophages with intranasal interferon gamma in a novel neonatal mouse model of respiratory syncytial virus infection. PLoS ONE. 2012;7(7):e40499. doi:10.1371/journal.pone.0040499.
Tasker L, Lindsay RW, Clarke BT, Cochrane DW, Hou S. Infection of mice with respiratory syncytial virus during neonatal life primes for enhanced antibody and T cell responses on secondary challenge. Clin Exp Immunol. 2008;153(2):277–88. doi:10.1111/j.1365-2249.2008.03591.x.
Han J, Dakhama A, Jia Y, Wang M, Zeng W, Takeda K et al. Responsiveness to respiratory syncytial virus in neonates is mediated through thymic stromal lymphopoietin and OX40 ligand. J Allergy Clin Immunol. 2012;130(5):1175-86 e9. doi:10.1016/j.jaci.2012.08.033.
Lee YM, Miyahara N, Takeda K, Prpich J, Oh A, Balhorn A, et al. IFN-gamma production during initial infection determines the outcome of reinfection with respiratory syncytial virus. Am J Respir Crit Care Med. 2008;177(2):208–18. doi:10.1164/rccm.200612-1890OC.
Ripple MJ, You D, Honnegowda S, Giaimo JD, Sewell AB, Becnel DM, et al. Immunomodulation with IL-4R alpha antisense oligonucleotide prevents respiratory syncytial virus-mediated pulmonary disease. J Immunol. 2010;185(8):4804–11. doi:10.4049/jimmunol.1000484.
You D, Marr N, Saravia J, Shrestha B, Lee GI, Turvey SE, et al. IL-4Ralpha on CD4+ T cells plays a pathogenic role in respiratory syncytial virus reinfection in mice infected initially as neonates. J Leukoc Biol. 2013;93(6):933–42. doi:10.1189/jlb.1012498.
Horvat JC, Beagley KW, Wade MA, Preston JA, Hansbro NG, Hickey DK, et al. Neonatal chlamydial infection induces mixed T-cell responses that drive allergic airway disease. Am J Respir Crit Care Med. 2007;176(6):556–64.
Jupelli M, Selby DM, Guentzel MN, Chambers JP, Forsthuber TG, Zhong G, et al. The contribution of interleukin-12/interferon-gamma axis in protection against neonatal pulmonary Chlamydia muridarum challenge. J Interferon Cytokine Res. 2010;30(6):407–15. doi:10.1089/jir 2009.0083.
Jupelli M, Guentzel MN, Meier PA, Zhong G, Murthy AK, Arulanandam BP. Endogenous IFN-gamma production is induced and required for protective immunity against pulmonary chlamydial infection in neonatal mice. J Immunol. 2008;180(6):4148–55.
Beckett EL, Phipps S, Starkey MR, Horvat JC, Beagley KW, Foster PS, et al. TLR2, but not TLR4, is required for effective host defence against Chlamydia respiratory tract infection in early life. PLoS ONE. 2012;7(6):e39460. doi:10.1371/journal.pone.0039460.
Jupelli M, Murthy AK, Chaganty BK, Guentzel MN, Selby DM, Vasquez MM, et al. Neonatal chlamydial pneumonia induces altered respiratory structure and function lasting into adult life. Lab Invest. 2011;91(10):1530–9. doi:10.1038/labinvest.2011.103.
Horvat JC, Starkey MR, Kim RY, Phipps S, Gibson PG, Beagley KW et al. Early-life chlamydial lung infection enhances allergic airways disease through age-dependent differences in immunopathology. J Allergy Clin Immunol. 2010;125(3):617–25, 25 e1–25 e6.
Principi N, Esposito S, Blasi F, Allegra L. Role of Mycoplasma pneumoniae and Chlamydia pneumoniae in children with community-acquired lower respiratory tract infections. Clin Infect Dis. 2001;32(9):1281–9. doi:10.1086/319981.
Hansbro PM, Beagley KW, Horvat JC, Gibson PG. Role of atypical bacterial infection of the lung in predisposition/protection of asthma. Pharmacol Ther. 2004;101(3):193–210. doi:10.1016/j.pharmthera.2003.10.007.
Webley WC, Salva PS, Andrzejewski C, Cirino F, West CA, Tilahun Y, et al. The bronchial lavage of pediatric patients with asthma contains infectious Chlamydia. Am J Respir Crit Care Med. 2005;171(10):1083–8. doi:10.1164/rccm.200407-917OC.
Webley WC, Tilahun Y, Lay K, Patel K, Stuart ES, Andrzejewski C, et al. Occurrence of Chlamydia trachomatis and Chlamydia pneumoniae in paediatric respiratory infections. Eur Respir J. 2009;33(2):360–7. doi:10.1183/09031936.00019508.
Procario MC, Levine RE, McCarthy MK, Kim E, Zhu L, Chang CH, et al. Susceptibility to acute mouse adenovirus type 1 respiratory infection and establishment of protective immunity in neonatal mice. J Virol. 2012;86(8):4194–203. doi:10.1128/JVI.06967-11.
Feil R, Fraga MF. Epigenetics and the environment: emerging patterns and implications. Nat Rev Genet. 2011;13(2):97–109. doi:10.1038/nrg3142.
Berger SL, Kouzarides T, Shiekhattar R, Shilatifard A. An operational definition of epigenetics. Genes Dev. 2009;23(7):781–3. doi:10.1101/gad.1787609.
Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. doi:10.1016/j.cell.2007.02.005.
Margueron R, Reinberg D. Chromatin structure and the inheritance of epigenetic information. Nat Rev Genet. 2010;11(4):285–96. doi:10.1038/nrg2752.
Rose S, Lichtenheld M, Foote M, Adkins B. Murine neonatal CD4+ cells are poised for rapid Th2 effector-like function. J Immunol. 2007;178(5):2667–78.
Yoshimoto M, Yoder MC, Guevara P, Adkins B. The murine Th2 locus undergoes epigenetic modification in the thymus during fetal and postnatal ontogeny. PLoS ONE. 2013;8(1):e51587. doi:10.1371/journal.pone.0051587.
Schoenborn JR, Dorschner MO, Sekimata M, Santer DM, Shnyreva M, Fitzpatrick DR, et al. Comprehensive epigenetic profiling identifies multiple distal regulatory elements directing transcription of the gene encoding interferon-gamma. Nat Immunol. 2007;8(7):732–42.
Martino D, Maksimovic J, Joo JH, Prescott SL, Saffery R. Genome-scale profiling reveals a subset of genes regulated by DNA methylation that program somatic T-cell phenotypes in humans. Genes Immun. 2012;. doi:10.1038/gene.2012.7.
Martino DJ, Tulic MK, Gordon L, Hodder M, Richman TR, Metcalfe J, et al. Evidence for age-related and individual-specific changes in DNA methylation profile of mononuclear cells during early immune development in humans. Epigenetics. 2011;6(9):1085–94. doi:10.4161/epi.6.9.16401.
White GP, Hollams EM, Yerkovich ST, Bosco A, Holt BJ, Bassami MR, et al. CpG methylation patterns in the IFN gamma promoter in naive T cells: variations during Th1 and Th2 differentiation and between atopics and non-atopics. Pediatr Allergy Immunol. 2006;17(8):557–64. doi:10.1111/j.1399-3038.2006.00465.x.
White GP, Watt PM, Holt BJ, Holt PG. Differential patterns of methylation of the IFN-gamma promoter at CpG and non-CpG sites underlie differences in IFN-gamma gene expression between human neonatal and adult CD45RO- T cells. J Immunol. 2002;168(6):2820–7.
Kaminuma O, Kitamura F, Miyatake S, Yamaoka K, Miyoshi H, Inokuma S et al. T-box 21 transcription factor is responsible for distorted T(H)2 differentiation in human peripheral CD4+ T cells. J Allergy Clin Immunol. 2009;123(4):813-23 e3. doi:10.1016/j.jaci.2009.01.055.
Jacoby M, Gohrbandt S, Clausse V, Brons NH, Muller CP. Interindividual variability and co-regulation of DNA methylation differ among blood cell populations. Epigenetics. 2012;7(12):1421–34. doi:10.4161/epi.22845.
Luo C, Burgeon E, Carew JA, McCaffrey PG, Badalian TM, Lane WS, et al. Recombinant NFAT1 (NFATp) is regulated by calcineurin in T cells and mediates transcription of several cytokine genes. Mol Cell Biol. 1996;16(7):3955–66.
Kiani A, Garcia-Cozar FJ, Habermann I, Laforsch S, Aebischer T, Ehninger G, et al. Regulation of interferon-gamma gene expression by nuclear factor of activated T cells. Blood. 2001;98(5):1480–8.
Chow CW, Rincon M, Davis RJ. Requirement for transcription factor NFAT in interleukin-2 expression. Mol Cell Biol. 1999;19(3):2300–7.
Kadereit S, Mohammad SF, Miller RE, Woods KD, Listrom CD, McKinnon K, et al. Reduced NFAT1 protein expression in human umbilical cord blood T lymphocytes. Blood. 1999;94(9):3101–7.
Weitzel RP, Lesniewski ML, Haviernik P, Kadereit S, Leahy P, Greco NJ, et al. microRNA 184 regulates expression of NFAT1 in umbilical cord blood CD4+ T cells. Blood. 2009;113(26):6648–57. doi:10.1182/blood-2008-09-181156.
Lederhuber H, Baer K, Altiok I, Sadeghi K, Herkner KR, Kasper DC. MicroRNA-146: tiny player in neonatal innate immunity? Neonatology. 2011;99(1):51–6. doi:10.1159/000301938.
Nahid MA, Pauley KM, Satoh M, Chan EK. miR-146a is critical for endotoxin-induced tolerance: implication in innate immunity. J Biol Chem. 2009;284(50):34590–9. doi:10.1074/jbc.M109.056317.
Nahid MA, Satoh M, Chan EK. Mechanistic role of microRNA-146a in endotoxin-induced differential cross-regulation of TLR signaling. J Immunol. 2011;186(3):1723–34. doi:10.4049/jimmunol.1002311.
Chassin C, Kocur M, Pott J, Duerr CU, Gutle D, Lotz M, et al. miR-146a mediates protective innate immune tolerance in the neonate intestine. Cell Host Microbe. 2010;8(4):358–68. doi:10.1016/j.chom.2010.09.005.
Huang HC, Yu HR, Huang LT, Chen RF, Lin IC, Ou CY, et al. miRNA-125b regulates TNF-alpha production in CD14+ neonatal monocytes via post-transcriptional regulation. J Leukoc Biol. 2012;92(1):171–82. doi:10.1189/jlb.1211593.
Palin AC, Ramachandran V, Acharya S, Lewis DB. Human neonatal naive CD4+ T cells have enhanced activation-dependent signaling regulated by the MicroRNA miR-181a. J Immunol. 2013;190(6):2682–91. doi:10.4049/jimmunol.1202534.
Goriely S, Van Lint C, Dadkhah R, Libin M, De Wit D, Demonte D et al. A defect in nucleosome remodeling prevents IL-12(p35) gene transcription in neonatal dendritic cells. J Exp Med. 2004;199(7):1011–6. doi:10.1084/jem.20031272.
Porras A, Kozar S, Russanova V, Salpea P, Hirai T, Sammons N, et al. Developmental and epigenetic regulation of the human TLR3 gene. Mol Immunol. 2008;46(1):27–36. doi:10.1016/j.molimm.2008.06.030.
Yancopoulos GD, Desiderio SV, Paskind M, Kearney JF, Baltimore D, Alt FW. Preferential utilization of the most JH-proximal VH gene segments in pre-B-cell lines. Nature. 1984;311(5988):727–33.
Perlmutter RM, Kearney JF, Chang SP, Hood LE. Developmentally controlled expression of immunoglobulin VH genes. Science. 1985;227(4694):1597–601.
Xu CR, Schaffer L, Head SR, Feeney AJ. Reciprocal patterns of methylation of H3K36 and H3K27 on proximal vs. distal IgVH genes are modulated by IL-7 and Pax5. Proc Natl Acad Sci USA. 2008;105(25):8685–90.
Garman RD, Doherty PJ, Raulet DH. Diversity, rearrangement, and expression of murine T cell gamma genes. Cell. 1986;45(5):733–42.
Goldman JP, Spencer DM, Raulet DH. Ordered rearrangement of variable region genes of the T cell receptor gamma locus correlates with transcription of the unrearranged genes. J Exp Med. 1993;177(3):729–39.
Heilig JS, Tonegawa S. Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. Nature. 1986;322(6082):836–40.
Agata Y, Katakai T, Ye SK, Sugai M, Gonda H, Honjo T, et al. Histone acetylation determines the developmentally regulated accessibility for T cell receptor gamma gene recombination. J Exp Med. 2001;193(7):873–80.
Hao B, Krangel MS. Long-distance regulation of fetal V(delta) gene segment TRDV4 by the Tcrd enhancer. J Immunol. 2011;187(5):2484–91. doi:10.4049/jimmunol.1100468.
Martino D, Prescott S. Epigenetics and prenatal influences on asthma and allergic airways disease. Chest. 2011;139(3):640–7. doi:10.1378/chest.10-1800.
Martino DJ, Prescott SL. Silent mysteries: epigenetic paradigms could hold the key to conquering the epidemic of allergy and immune disease. Allergy. 2009;65(1):7–15. doi:10.1111/j.1398-9995.2009.02186.x.
Pfefferle PI, Pinkenburg O, Renz H. Fetal epigenetic mechanisms and innate immunity in asthma. Curr Allergy Asthma Rep. 2010;10(6):434–43. doi:10.1007/s11882-010-0147-6.
North ML, Ellis AK. The role of epigenetics in the developmental origins of allergic disease. Ann Allergy Asthma Immunol. 2011;106(5):355-61; quiz 62. doi:10.1016/j.anai.2011.02.008.
Breton CV, Byun HM, Wenten M, Pan F, Yang A, Gilliland FD. Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation. Am J Respir Crit Care Med. 2009;180(5):462–7. doi:10.1164/rccm.200901-0135OC.
Bobetsis YA, Barros SP, Lin DM, Weidman JR, Dolinoy DC, Jirtle RL, et al. Bacterial infection promotes DNA hypermethylation. J Dent Res. 2007;86(2):169–74. doi:10.1177/154405910708600212.
Hollingsworth JW, Maruoka S, Boon K, Garantziotis S, Li Z, Tomfohr J, et al. In utero supplementation with methyl donors enhances allergic airway disease in mice. J Clin Invest. 2008;118(10):3462–9. doi:10.1172/JCI34378.
Schaub B, Liu J, Hoppler S, Schleich I, Huehn J, Olek S et al. Maternal farm exposure modulates neonatal immune mechanisms through regulatory T cells. J Allergy Clin Immunol. 2009;123(4):774-82 e5. doi:10.1016/j.jaci.2009.01.056.
Vuillermin PJ, Ponsonby AL, Saffery R, Tang ML, Ellis JA, Sly P, et al. Microbial exposure, interferon gamma gene demethylation in naive T-cells, and the risk of allergic disease. Allergy. 2009;64(3):348–53. doi:10.1111/j.1398-9995.2009.01970.x.
Perera F, Tang WY, Herbstman J, Tang D, Levin L, Miller R, et al. Relation of DNA methylation of 5′-CpG island of ACSL3 to transplacental exposure to airborne polycyclic aromatic hydrocarbons and childhood asthma. PLoS ONE. 2009;4(2):e4488. doi:10.1371/journal.pone.0004488.
Tang WY, Levin L, Talaska G, Cheung YY, Herbstman J, Tang D, et al. Maternal exposure to polycyclic aromatic hydrocarbons and 5′-CpG methylation of interferon-gamma in cord white blood cells. Environ Health Perspect. 2012;120(8):1195–200. doi:10.1289/ehp.1103744.
Haberg SE, London SJ, Stigum H, Nafstad P, Nystad W. Folic acid supplements in pregnancy and early childhood respiratory health. Arch Dis Child. 2009;94(3):180–4. doi:10.1136/adc.2008.142448.
Whitrow MJ, Moore VM, Rumbold AR, Davies MJ. Effect of supplemental folic acid in pregnancy on childhood asthma: a prospective birth cohort study. Am J Epidemiol. 2009;170(12):1486–93. doi:10.1093/aje/kwp315.
Fedulov AV, Kobzik L. Allergy risk is mediated by dendritic cells with congenital epigenetic changes. Am J Respir Cell Mol Biol. 2011;44(3):285–92. doi:10.1165/rcmb.2009-0400OC.
Brand S, Teich R, Dicke T, Harb H, Yildirim AO, Tost J et al. Epigenetic regulation in murine offspring as a novel mechanism for transmaternal asthma protection induced by microbes. J Allergy Clin Immunol. 2011;128(3):618-25 e1-7. doi:10.1016/j.jaci.2011.04.035.
Braun-Fahrlander C, Riedler J, Herz U, Eder W, Waser M, Grize L, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347(12):869–77. doi:10.1056/NEJMoa020057.
Ege MJ, Bieli C, Frei R, van Strien RT, Riedler J, Ublagger E, et al. Prenatal farm exposure is related to the expression of receptors of the innate immunity and to atopic sensitization in school-age children. J Allergy Clin Immunol. 2006;117(4):817–23. doi:10.1016/j.jaci.2005.12.1307.
Douwes J, Cheng S, Travier N, Cohet C, Niesink A, McKenzie J, et al. Farm exposure in utero may protect against asthma, hay fever and eczema. Eur Respir J. 2008;32(3):603–11. doi:10.1183/09031936.00033707.
Acknowledgments
Supported by National Institute of Allergy and Infectious Diseases grants AI044923 and AI083461.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adkins, B. Neonatal immunology: responses to pathogenic microorganisms and epigenetics reveal an “immunodiverse” developmental state. Immunol Res 57, 246–257 (2013). https://doi.org/10.1007/s12026-013-8439-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-013-8439-2