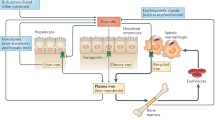
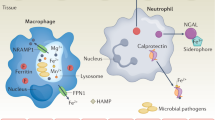
Abstract
My laboratory has been interested for some time in the influence of iron, a nutrient that is essential for both microbial pathogens and their mammalian hosts, on the course of infectious disease. Our studies indicate that alterations in the expression of host molecules that sequester or transport iron can have direct effects on pathogen growth and can also have an impact on the ability to mount normal immune responses. We have elucidated the mechanistic basis for some of these observations, and have started to apply our findings in strategies to control abnormalities of inflammation and iron metabolism. I will review here what we have learned about the interactions between iron and immunity and discuss the implications of the information that we have acquired.


Similar content being viewed by others
References
Beard JL. Iron biology in immune function, muscle metabolism and neuronal functioning. J Nutr. 2001;131:568S–80S.
Andrews NC. Forging a field: the golden age of iron biology. Blood. 2008;112:219–30.
Hentze MW, Muckenthaler MU, Galy B, et al. Two to tango: regulation of mammalian iron metabolism. Cell. 2010;142:24–38.
Biggs TE, Baker ST, Botham MS, et al. Nramp1 modulates iron homeostasis in vivo and in vitro: evidence for a role in cellular iron release involving de-acidification of intracellular vesicles. Eur J Immunol. 2001;31:2060–70.
Soe-Lin S, Sheftel AD, Wasyluk B, et al. Nramp1 equips macrophages for efficient iron recycling. Exp Hematol. 2008;36:929–37.
Soe-Lin S, Apte SS, Andriopoulos B Jr, et al. Nramp1 promotes efficient macrophage recycling of iron following erythrophagocytosis in vivo. Proc Natl Acad Sci USA. 2009;106:5960–5.
Feder JN, Gnirke A, Thomas W, et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996;13:399–408.
Goswami T, Andrews NC. Hereditary hemochromatosis protein, HFE, interaction with transferrin receptor 2 suggests a molecular mechanism for mammalin iron sensing. J Biol Chem. 2006;281:28494–8.
Schmidt PJ, Toran PT, Giannetti AM, et al. The transferrin receptor modulates Hfe-dependent regulation of hepcidin expression. Cell Metab. 2008;7:205–14.
Gao J, Chen J, Kramer M, et al. Interaction of the hereditary hemochromatosis protein, HFE, with transferrin receptor 2 is required for transferrin-induced hepcidin expression. Cell Metab. 2009;9:217–27.
Andriopoulos B, Corradini E, Xia Y, et al. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. 2009;41:482–7.
Meynard D, Kautz L, Darnaud V, et al. Lack of BMP6 induces massive iron overload. Nat Genet. 2009;41:478–81.
Ganz T, Nemeth E. Hepcidin and disorders of iron metabolism. Annu Rev Med. 2011;62:13.1–4. (epub ahead of print).
Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–3.
De Domenico I, Ward DM, Langelier C, et al. The molecular mechanism of hepcidin-mediated ferroportin down-regulation. Mol Biol Cell. 2007;18:2569–78.
De Domenico I, Lo E, Ward DM, et al. Hepcidin-induced internalization of ferroportin requires binding and cooperative interaction with Jak2. Proc Natl Acad Sci USA. 2009;106:3800–5.
Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–6.
Lee P, Peng H, Gelbart T, Beutler E. The IL-6 and LPS-induced transcription of hepcidin in Hfe- TfR2- and β2-microglobulin-deficient mice. Proc Natl Acad Sci USA. 2004;101:9263–5.
Lee P, Peng H, Gelbart T, et al. Regulation of hepcidin transcription by IL-1 and IL-6. Proc Natl Acad Sci USA. 2005;102:1906–10.
Muckenthaler MU, Galy B, Hentze MW. Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu Rev Nutr. 2008;28:197–213.
Wang L, Cherayil BJ. Ironing out the wrinkles in host defense: interactions between iron homeostasis and innate immunity. J Innate Immun. 2009;1:455–64.
Forbes JR, Gros P. Divalent metal transport by NRAMP proteins at the interface of host-pathogen interactions. Trends Microbiol. 2001;9:397–403.
Cellier MF, Courville P, Campion C. Nramp1 phagocyte intracellular metal withdrawal defense. Microbes Infect. 2007;9:1662–70.
Moller M, Hoal EG. Current findings, challenges and novel approaches to human genetic susceptibility to tuberculosis. Tuberculosis (Edinb). 2010;90:71–83.
Chlosta S, Fishman DS, Harrington L, et al. The iron efflux protein ferroportin regulates the intracellular growth of Salmonella enterica. Infect Immun. 2006;74:3065–7.
Olakanmi O, Schlesinger LS, Britigan BE. Hereditary hemochromatosis results in decreased iron acquisition and growth by Mycobacterium tuberculosis within human macrophages. J Leukoc Biol. 2007;81:195–204.
Nairz M, Theurl I, Ludwiczek S, et al. The co-ordinated regulation of iron homeostasis in murine macrophages limits the availability of iron for intracellular Salmonella typhimurium. Cell Microbiol. 2007;9:2126–40.
Paradkar PN, De Domenico I, Durchfort N, et al. Iron depletion limits intracellular bacterial growth in macrophages. Blood. 2008;112:866–74.
Nairz M, Fritsche G, Brunner P, et al. Interferon γ limits the availability of iron for intra-macrophage Salmonella typhimurium. Eur J Immunol. 2008;38:1923–36.
Peyssonaux C, Zinkernagel AS, Datta V, et al. TLR4-dependent hepcidin expression by myeloid cells in response to bacterial pathogens. Blood. 2006;107:3727–32.
Nguyen NB, Callaghan KD, Ghio AJ, et al. Hepcidin expression and iron transport in alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2006;291:L417–25.
Theurl I, Theurl M, Seifert M, et al. Autocrine formation of hepcidin induces iron retention in human monocytes. Blood. 2008;111:2392–9.
Schaible UE, Kaufmann SH. Iron and microbial infection. Nat Rev Microbiol. 2004;2:946–53.
Doherty CP. Host-pathogen interactions: the role of iron. J Nutr. 2007;137:1341–4.
Pietrangelo A. Hereditary hemochromatosis: pathogenesis, diagnosis, and treatment. Gastroenterology. 2010;139:393–408.
Zhou XY, Tomatsu S, Fleming RE, et al. Hfe gene knock-out produces a mouse model of hereditary hemochromatosis. Proc Natl Acad Sci USA. 1998;95:2492–7.
Wang L, Johnson EE, Shi HN, et al. Attenuated inflammatory responses in hemochromatosis reveal a role for iron in the regulation of macrophage cytokine translation. J Immunol. 2008;181:2723–31.
Gomes-Pereira S, Rodrigues PN, Appelberg R, et al. Increased susceptibility to Mycobacterium avium in hemochromatosis protein Hfe-deficient mice. Infect Immun. 2008;76:4713–9.
Johnson EE, Sandgren A, Cherayil BJ, et al. Role of ferroportin in macrophage-mediated immunity. Infect Immun. 2010;78:5099–106.
Wang L, Harrington L, Trebicka E, et al. Selective modulation of TLR4-activated inflammatory responses by altered iron homeostasis in mice. J Clin Invest. 2009;119:3322–8.
Sheedy FJ, O’Neill LA. The Troll in Toll: Mal and TRAM as bridges for TLR2 and TLR4 signaling. J Leukoc Biol. 2007;82:196–203.
Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–84.
Kagan JC, Su T, Horng T, et al. TRAM couples endocytosis of TLR4 to the induction of interferon-beta. Nat Immunol. 2008;9:361–8.
Nairz M, Theurl I, Schroll A, et al. Absence of functional Hfe protects mice from invasive Salmonella enterica serovar Typhimurium infection via induction of lipocalin-2. Blood. 2009;114:3642–51.
Levy JE, Montross LK, Cohen DE, et al. The C282Y mutation causing hereditary hemochromatosis does not produce a null allele. Blood. 1999;94:9–11.
Bahram S, Gilfillan S, Kuhn LC, et al. Experimental hemochromatosis due to MHC class I HFE deficiency: immune status and iron metabolism. Proc Natl Acad Sci USA. 1999;96:13312–7.
De Domenico I, Zhang TY, Branch LW, et al. Hepcidin mediates transcriptional changes that modulate acute cytokine-induced inflammatory responses in mice. J Clin Invest. 2010;120:2395–405.
Bullen JJ, Spalding PB, Ward CG, et al. Hemochromatosis, iron and septicemia caused by Vibrio vulnificus. Arch Intern Med. 1991;151:1606–9.
Bergmann TK, Vinding K, Hey H. Multiple hepatic abscesses due to Yersinia enterocolitica infection secondary to primary hemochromatosis. Scand J Gastroenterol. 2001;36:891–5.
Wright AC, Simpson LM, Oliver JD. Role of iron in the pathogenesis of Vibrio vulnificus infections. Infect Immun. 1981;34:503–7.
Gordeuk VR, Ballou S, Lozanski G, et al. Decreased concentrations of TNFα in supernatants of monocytes from homozygotes for hereditary hemochromatosis. Blood. 1992;79:1855–60.
Gordeuk VR, Caleffi A, Corradini E, et al. Iron overload in Africans and African-Americans and a common mutation in the SLC40A1 (ferroportin 1) gene. Blood Cells Mol Dis. 2003;31:299–304.
Beutler E, Barton JC, Felitti VJ, et al. Ferroportin 1 (SLC40A1) variant associated with iron overload in African-Americans. Blood Cells Mol Dis. 2003;31:305–9.
McNamara L, Gordeuk VR, MacPhail AP. Ferroportin (Q248H) mutations in African families with dietary iron overload. J Gastroenterol Hepatol. 2005;20:1855–8.
Boelaert JR, Vandecasteele SJ, Appelberg R, et al. The effect of the host’s iron status on tuberculosis. J Infect Dis. 2007;195:1745–53.
Gomolion F, Gisbert JP. Anemia and inflammatory bowel diseases. World J Gastroenterol. 2009;15:4659–65.
Stein J, Hartmann F, Dignass AU. Diagnosis and management of iron deficiency anemia in patients with IBD. Nat Rev Gastroenterol Hepatol. 2010;7:599–610.
Ganz T, Nemeth E. Iron sequestration and anemia of inflammation. Semin Hematol. 2009;46:387–93.
Wessling-Resnick M. Iron homeostasis and the inflammatory response. Annu Rev Nutr. 2010;30:105–22.
Semrin G, Fishman DS, Bousvaros A, et al. Impaired intestinal iron absorption in Crohn’s disease correlates with disease activity and markers of inflammation. Inflamm Bowel Dis. 2006;12:1101–6.
Cherayil BJ. Cross-talk between iron homeostasis and intestinal inflammation. Gut Microbes. 2010;1:65–9.
Babitt JL, Huang FW, Xia Y, et al. Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance. J Clin Invest. 2007;117:1933–9.
Yu PB, Hong CC, Sachidanandan C, et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41.
Berg DJ, Zhang J, Weinstock JV, et al. Rapid development of colitis in NSAID-treated IL-10-deficient mice. Gastroenterology. 2002;123:1527–42.
Legrand D, Mazurier J. A critical review of the roles of host lactoferrin in immunity. Biometals. 2010;23:365–76.
Ward PP, Mendoza-Meneses M, Park PW, et al. Stimulus-dependent impairment of neutrophil oxidative burst response in lactoferrin-deficient mice. Am J Pathol. 2008;172:1019–29.
Clifton MC, Corrent C, Strong RK. Siderocalins: siderophore-binding proteins of the innate immune system. Biometals. 2009;22:557–64.
Goetz DH, Holmes MA, Borregaard N, et al. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10:1033–43.
Miethke M, Marahiel MA. Siderophore-based iron acquisition and pathogen contol. Microbiol Mol Biol Rev. 2007;71:413–51.
Flo TH, Smith KD, Sato S, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–21.
Berger T, Togawa A, Duncan GS, et al. Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2006;103:1834–9.
Chan YR, Liu JS, Pociask DA, et al. Lipocalin 2 is required for pulmonary host defense against Klebsiella infection. J Immunol. 2009;182:4947–56.
Fischbach MA, Lin H, Liu DR, et al. How pathogenic bacteria evade mammalian sabotage in the battle for iron. Nat Chem Biol. 2006;2:132–8.
Fischbach MA, Lin H, Zhou L, et al. The pathogen-associated iroA gene cluster mediates bacterial evasion of lipocalin 2. Proc Natl Acad Sci USA. 2006;103:16502–7.
Raffatellu M, George MD, Akiyama Y, et al. Lipocalin 2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe. 2009;5:476–86.
Holmes MA, Paulsene W, Jide X, et al. Siderocalin (Lcn2) also binds carboxymycobactins, potentially defending against mycobacterial infections through iron sequestration. Structure. 2005;13:29–41.
Saiga H, Nishimura J, Kuwata H, et al. Lipocalin 2-dependent inhibition of mycobacterial growth in alveolar epithelium. J Immunol. 2008;181:8521–7.
Johnson EE, Srikanth CV, Sandgren A, et al. Siderocalin inhibits the intracellular replication of Mycobacterium tuberculosis in macrophages. FEMS Immunol Med Microbiol. 2010;58:138–45.
Martineau AR, Newton SM, Wilkinson KA, et al. Neutrophil-mediated innate immune resistance to mycbacteria. J Clin Invest. 2007;117:1988–94.
Landro L, Damas JK, Flo TH, et al. Decreased serum lipocalin 2 levels in human immunodeficiency virus-infected patients: increase during highly active anti-retroviral therapy. Clin Exp Immunol. 2008;152:57–63.
Devireddy LR, Hart DO, Goetz DH, et al. A mammalian siderophore synthesized by an enzyme with a bacterial homolog involved in enterobactin production. Cell. 2010;141:1006–17.
Bao G, Clifton M, Hoette TM, et al. Iron traffics in circulation bound to a siderocalin (NGAL)-catechol complex. Nat Chem Biol. 2010;6:602–9.
Philpott C. Bioinorganic chemistry: getting a grip on iron. Nat Chem Biol. 2010;6:568–70.
Yang J, Goetz D, Li JY, et al. An iron delivery pathway mediated by lipocalin. Mol Cell. 2002;10:1045–56.
Kaplan J. Mechanisms of cellular iron acquisition: another iron in the fire. Cell. 2002;111:603–6.
Devireddy LR, Gazin C, Zhu X, et al. A cell surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell. 2005;123:1293–305.
Richardson DR. 24p3 and its receptor: dawn of a new iron age? Cell. 2005;123:1175–7.
Breuer W, Shvartsman M. Cabantchik ZI: Intracellular labile iron. Int J Biochem Cell Biol. 2008;40:350–4.
Kell DB. Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular, other progressive inflammatory, degenerative diseases. BMC Med Genomics. 2009;2:2.
Kell DB. Towards a unifying systems biology understanding of large-scale cellular death and destruction caused by poorly liganded iron: Parkinson’s, Huntington’s, Alzheimer’s, prions, bactericides, chemical toxicology and others as examples. Arch Toxicol. 2010;84:825–89.
Baker M, Wilson D, Sabeti PC, et al. Host genetic factors involved in iron regulation may influence susceptibility to Mycobacterium tuberculosis: a pilot study. Abstract presented at the Fourth Annual New England TB Symposium, July 2010.
Acknowledgments
Work in the author’s laboratory is supported by grants from the National Institutes of Health (R56AI089700), the Broad Medical Research Program (IBD-0253) and the Crohn’s and Colitis Foundation of America (1754).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cherayil, B.J. The role of iron in the immune response to bacterial infection. Immunol Res 50, 1–9 (2011). https://doi.org/10.1007/s12026-010-8199-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-010-8199-1