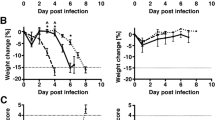
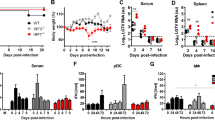
Abstract
Neuroinflammation, including astrogliosis, microgliosis, and the production of proinflammatory cytokines and chemokines is a common response in the central nervous system (CNS) to virus infection, including retrovirus infection. However, the contribution of this innate immune response in disease pathogenesis remains unresolved. Analysis of the neuroinflammatory response to polytropic retrovirus infection in the mouse has provided insight into the potential contribution of the innate immune response to retrovirus-induced neurologic disease. In this model, retroviral pathogenesis correlates with the induction of neuroinflammatory responses including the activation of astrocytes and microglia, as well as the production of proinflammatory cytokines and chemokines. Studies of the neurovirulent determinants of the polytropic envelope protein as well as studies with knockout mice suggest that retroviral pathogenesis in the brain is multifaceted and that cytokine and chemokine production may be only one mechanism of disease pathogenesis. Analysis of the activation of the innate immune response to retrovirus infection in the CNS indicates that toll-like receptor 7 (TLR7) is a contributing factor to retrovirus-induced neuroinflammation, but that other factors can compensate for the lack of TLR7 in inducing both neuroinflammation and neurologic disease.
Similar content being viewed by others
References
Dickson DW, Llena JF, Nelson SJ, Weidenheim KM. Central nervous system pathology in pediatric AIDS. Ann N Y Acad Sci. 1993;693:93–106.
Kolson DL, Lavi E, Gonzalez-Scarano F. The effects of human immunodeficiency virus in the central nervous system. Adv Virus Res. 1998;50:1–47.
McCoig C, Castrejon MM, Saavedra-Lozano J, Castano E, Baez C, Lanier ER, et al. Cerebrospinal fluid and plasma concentrations of proinflammatory mediators in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 2004;23(2):114–8.
Tyor WR, Glass JD, Griffin JW, Becker PS, McArthur JC, Bezman L, et al. Cytokine expression in the brain during the acquired immunodeficiency syndrome. Ann Neurol. 1992;31(4):349–60.
Vago L, Nebuloni M, Bonetto S, Pellegrinelli A, Zerbi P, Ferri A, et al. Rantes distribution and cellular localization in the brain of HIV-infected patients. Clin Neuropathol. 2001;20(4):139–45.
Askovic S, Favara C, McAtee FJ, Portis JL. Increased expression of MIP-1 alpha and MIP-1 beta mRNAs in the brain correlates spatially and temporally with the spongiform neurodegeneration induced by a murine oncornavirus. J Virol. 2001;75(6):2665–74.
Choe W, Stoica G, Lynn W, Wong PK. Neurodegeneration induced by MoMuLV-ts1 and increased expression of Fas and TNF-alpha in the central nervous system. Brain Res. 1998;779(1–2):1–8.
Orandle MS, MacLean AG, Sasseville VG, Alvarez X, Lackner AA. Enhanced expression of proinflammatory cytokines in the central nervous system is associated with neuroinvasion by simian immunodeficiency virus and the development of encephalitis. J Virol. 2002;76(11):5797–802.
Peterson KE, Robertson SJ, Portis JL, Chesebro B. Differences in cytokine and chemokine responses during neurological disease induced by polytropic murine retroviruses Map to separate regions of the viral envelope gene. J Virol. 2001;75(6):2848–56.
Poli A, Abramo F, Di Iorio C, Cantile C, Carli MA, Pollera C, et al. Neuropathology in cats experimentally infected with feline immunodeficiency virus: a morphological, immunocytochemical and morphometric study. J Neurovirol. 1997;3(5):361–8.
Poli A, Pistello M, Carli MA, Abramo F, Mancuso G, Nicoletti E, et al. Tumor necrosis factor-alpha and virus expression in the central nervous system of cats infected with feline immunodeficiency virus. J Neurovirol. 1999;5(5):465–73.
Westmoreland SV, Rottman JB, Williams KC, Lackner AA, Sasseville VG. Chemokine receptor expression on resident and inflammatory cells in the brain of macaques with simian immunodeficiency virus encephalitis. Am J Pathol. 1998;152(3):659–65.
Zink MC, Coleman GD, Mankowski JL, Adams RJ, Tarwater PM, Fox K, et al. Increased macrophage chemoattractant protein-1 in cerebrospinal fluid precedes and predicts simian immunodeficiency virus encephalitis. J Infect Dis. 2001;184(8):1015–21.
Barker CF, Billingham RE. Immunologically privileged sites. Adv Immunol. 1977;25:1–54.
Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix CC. CNS immune privilege: hiding in plain sight. Immunol Rev. 2006;213:48–65.
Saunders NR, Knott GW, Dziegielewska KM. Barriers in the immature brain. Cell Mol Neurobiol. 2000;20(1):29–40.
Perry VH, Hume DA, Gordon S. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience. 1985;15(2):313–26.
Perry VH, Gordon S. Macrophages and microglia in the nervous system. Trends Neurosci. 1988;11(6):273–7.
Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239(4837):290–2.
Lassmann H, Hickey WF. Radiation bone marrow chimeras as a tool to study microglia turnover in normal brain and inflammation. Clin Neuropathol. 1993;12(5):284–5.
Dickson DW, Lee SC, Mattiace LA, Yen SH, Brosnan C. Microglia and cytokines in neurological disease, with special reference to AIDS and Alzheimer’s disease. Glia. 1993;7(1):75–83.
Minagar A, Shapshak P, Fujimura R, Ownby R, Heyes M, Eisdorfer C. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J Neurol Sci. 2002;202(1–2):13–23.
Prat A, Biernacki K, Wosik K, Antel JP. Glial cell influence on the human blood–brain barrier. Glia. 2001;36(2):145–55.
Rosenberg PA, Aizenman E. Hundred-fold increase in neuronal vulnerability to glutamate toxicity in astrocyte-poor cultures of rat cerebral cortex. Neurosci Lett. 1989;103(2):162–8.
Rosenberg PA. Accumulation of extracellular glutamate and neuronal death in astrocyte-poor cortical cultures exposed to glutamine. Glia. 1991;4(1):91–100.
Magistretti PJ, Pellerin L, Rothman DL, Shulman RG. Energy on demand. Science. 1999;283(5401):496–7.
Mucke L, Eddleston M. Astrocytes in infectious and immune-mediated diseases of the central nervous system. FASEB J. 1993;7(13):1226–32.
Portis JL, Czub S, Robertson S, McAtee F, Chesebro B. Characterization of a neurologic disease induced by a polytropic murine retrovirus: evidence for differential targeting of ecotropic and polytropic viruses in the brain. J Virol. 1995;69(12):8070–5.
Robertson SJ, Hasenkrug KJ, Chesebro B, Portis JL. Neurologic disease induced by polytropic murine retroviruses: neurovirulence determined by efficiency of spread to microglial cells. J Virol. 1997;71(7):5287–94.
Battini JL, Rasko JE, Miller AD. A human cell-surface receptor for xenotropic and polytropic murine leukemia viruses: possible role in G protein-coupled signal transduction. Proc Natl Acad Sci USA. 1999;96(4):1385–90.
Wu T, Yan Y, Kozak CA. Rmcf2, a xenotropic provirus in the Asian mouse species Mus castaneus, blocks infection by polytropic mouse gammaretroviruses. J Virol. 2005;79(15):9677–84.
Yan Y, Knoper RC, Kozak CA. Wild mouse variants of envelope genes of xenotropic/polytropic mouse gammaretroviruses and their XPR1 receptors elucidate receptor determinants of virus entry. J Virol. 2007;81(19):10550–7.
Van Hoeven NS, Miller AD. Use of different but overlapping determinants in a retrovirus receptor accounts for non-reciprocal interference between xenotropic and polytropic murine leukemia viruses. Retrovirology. 2005;2:76.
DesGroseillers L, Barrette M, Jolicoeur P. Physical mapping of the paralysis-inducing determinant of a wild mouse ecotropic neurotropic retrovirus. J Virol. 1984;52(2):356–63.
Park BH, Matuschke B, Lavi E, Gaulton GN. A point mutation in the env gene of a murine leukemia virus induces syncytium formation and neurologic disease. J Virol. 1994;68(11):7516–24.
Portis JL, Czub S, Garon CF, McAtee FJ. Neurodegenerative disease induced by the wild mouse ecotropic retrovirus is markedly accelerated by long terminal repeat and gag-pol sequences from nondefective Friend murine leukemia virus. J Virol. 1990;64(4):1648–56.
Szurek PF, Floyd E, Yuen PH, Wong PK. Site-directed mutagenesis of the codon for Ile-25 in gPr80env alters the neurovirulence of ts1, a mutant of Moloney murine leukemia virus TB. J Virol. 1990;64(11):5241–9.
Yuen PH, Malehorn D, Nau C, Soong MM, Wong PK. Molecular cloning of two paralytogenic, temperature-sensitive mutants, ts1 and ts7, and the parental wild-type Moloney murine leukemia virus. J Virol. 1985;54(1):178–85.
Corbin ME, Pourciau S, Morgan TW, Boudreaux M, Peterson KE. Ligand up-regulation does not correlate with a role for CCR1 in pathogenesis in a mouse model of non-lymphocyte mediated neurological disease. J Neurovirol. 2006;12:1–10.
Peterson KE, Chesebro B. Influence of proinflammatory cytokines and chemokines on the neuropathogenesis of oncornavirus and immunosuppressive lentivirus infections. Curr Top Microbiol Immunol. 2006;303:67–95.
Lewis SD, Butchi NB, Khaleduzzaman M, Morgan TW, Du M, Pourciau S, et al. Toll-like receptor 7 is not necessary for retroviral neuropathogenesis but does contribute to viral-induced neuroinflammation. J Neurovirol. 2008; in press.
Peterson KE, Errett JS, Wei T, Dimcheff DE, Ransohoff R, Kuziel WA, et al. MCP-1 and CCR2 contribute to non-lymphocyte-mediated brain disease induced by Fr98 polytropic retrovirus infection in mice: role for astrocytes in retroviral neuropathogenesis. J Virol. 2004;78(12):6449–58.
Peterson KE, Pourciau S, Du M, Lacasse R, Pathmajeyan M, Poulsen D, et al. Neurovirulence of polytropic murine retrovirus is influenced by two separate regions on opposite sides of the envelope protein receptor binding domain. J Virol. 2008;82(17):8906–10.
Peterson KE, Hughes S, Dimcheff DE, Wehrly K, Chesebro B. Separate sequences in a murine retroviral envelope protein mediate neuropathogenesis by complementary mechanisms with differing requirements for tumor necrosis factor alpha. J Virol. 2004;78(23):13104–12.
Peterson KE, Evans LH, Wehrly K, Chesebro B. Increased proinflammatory cytokine and chemokine responses and microglial infection following inoculation with neural stem cells infected with polytropic murine retroviruses. Virology. 2006;354(1):143–53.
Hasenkrug KJ, Robertson SJ, Porti J, McAtee F, Nishio J, Chesebro B. Two separate envelope regions influence induction of brain disease by a polytropic murine retrovirus (FMCF98). J Virol. 1996;70(7):4825–8.
Poulsen DJ, Robertson SJ, Favara CA, Portis JL, Chesebro BW. Mapping of a neurovirulence determinant within the envelope protein of a polytropic murine retrovirus: induction of central nervous system disease by low levels of virus. Virology. 1998;248(2):199–207.
Banisadr G, Queraud-Lesaux F, Boutterin MC, Pelaprat D, Zalc B, Rostene W, et al. Distribution, cellular localization and functional role of CCR2 chemokine receptors in adult rat brain. J Neurochem. 2002;81(2):257–69.
van Gassen KL, Netzeband JG, de Graan PN, Gruol DL. The chemokine CCL2 modulates Ca dynamics and electrophysiological properties of cultured cerebellar Purkinje neurons. Eur J NeuroSci. 2005;21(11):2949–57.
White FA, Sun J, Waters SM, Ma C, Ren D, Ripsch M, et al. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc Natl Acad Sci USA. 2005;102(39):14092–7.
Janeway CA Jr. The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today. 1992;13(1):11–6.
Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2(8):675–80.
Beignon AS, McKenna K, Skoberne M, Manches O, DaSilva I, Kavanagh DG, et al. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest. 2005;115(11):3265–75.
Bochud PY, Hersberger M, Taffe P, Bochud M, Stein CM, Rodrigues SD, et al. Polymorphisms in Toll-like receptor 9 influence the clinical course of HIV-1 infection. AIDS. 2007;21(4):441–6.
Burzyn D, Rassa JC, Kim D, Nepomnaschy I, Ross SR, Piazzon I. Toll-like receptor 4-dependent activation of dendritic cells by a retrovirus. J Virol. 2004;78(2):576–84.
Butchi NB, Pourciau S, Du M, Morgan TW, Peterson KE. Analysis of the neuroinflammatory response to TLR7 stimulation in the brain: comparison of multiple TLR7 and/or TLR8 agonists. J Immunol. 2008;180(11):7604–12.
Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3(2):196–200.
Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW, et al. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci USA. 2004;101(15):5598–603.
Ciardi A, Sinclair E, Scaravilli F, Harcourt-Webster NJ, Lucas S. The involvement of the cerebral cortex in human immunodeficiency virus encephalopathy: a morphological and immunohistochemical study. Acta Neuropathol. 1990;81(1):51–9.
Acknowledgment
This research was supported in part by the Intramural Research Program of the NIH.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peterson, K.E., Du, M. Innate immunity in the pathogenesis of polytropic retrovirus infection in the central nervous system. Immunol Res 43, 149–159 (2009). https://doi.org/10.1007/s12026-008-8060-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-008-8060-y