Abstract
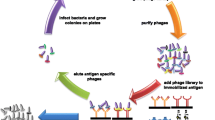
Recent developments in molecular immunology have facilitated the expression of immune repertoires in the form of immunoglobulin fragments on the surface of filamentous bacteriophage. Such approaches, known as “phage display”, have provided powerful tools for producing monoclonal antibodies for research, clinical, and therapeutic applications. Our laboratory has combined these techniques with novel selection methods to isolate extraordinarily large arrays of human antibodies that can be used for developing new diagnostic reagents and methods for their use, as well as reagents that may serve as leads for the design of novel therapeutic molecules. In particular, application of phage display to the study of antibody-mediated autoimmune disease, notably idiopathic thrombocytopenic purpura, thrombotic thrombocytopenic purpura, and pemphigus have facilitated comprehensive genetic and serologic analyses of pathogenic autoantibodies thereby offering new insights into disease pathophysiology and approaches for their treatment.







Similar content being viewed by others
References
Siegel DL. The human immune response to red blood cell antigens as revealed by repertoire cloning. Immunol Res. 1998;17:239–51.
Westhoff CM, Siegel DL. Rh and LW blood group systems. In: Simon TL, Snyder EL, Solheim BG, Stowell CP, Strauss RG, Petrides M, editors. Rossi’s principles of transfusion medicine. Bethesda: AABB; 2009 (in press).
Siegel DL, Chang TY, Russell SL, Bunya VY. Isolation of cell surface-specific human monoclonal antibodies using phage display and magnetically-activated cell sorting: applications in immunohematology. J Immunol Methods. 1997;206:73–85.
Chang TY, Siegel DL. Genetic and immunological properties of phage-displayed human anti-Rh(D) antibodies: implications for Rh(D) epitope topology. Blood. 1998;91:3066–78.
Chang TY, Siegel DL. Isolation of an IgG anti-B from a human Fab-phage display library. Transfusion. 2001;41:6–12.
Siegel DL. Research and clinical applications of antibody phage display in transfusion medicine. Transfus Med Rev. 2001;15:35–52.
Siegel DL. Recombinant monoclonal antibody technology. Transfus Clin Biol. 2002;9:15–22.
Siegel DL. Diagnostic and therapeutic applications of phage display technology. In: Stowell CP, Dzik WH, editors. Emerging technologies and therapies in transfusion medicine. Bethesda: AABB; 2003. p. 55–93.
Barbas C, Burton D, Silverman G, Scott J. Phage display of proteins and peptides: a laboratory manual. Cold Spring Harbor: Cold Spring Harbor Press; 2000.
Aitken R, O’Brien PM. Antibody phage display: methods and protocols. In: Methods in molecular biology, vol. 178. Totowa, NJ: Humana Press; 2002.
Clackson T, Lowman H. Phage display: a practical approach. New York: Oxford University Press; 2004.
Sidhu SS. Phage display in biotechnology and drug discovery. Boca Raton: CRC Press; 2005.
Smith GP. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985;228:1315–7.
Burton DR, Barbas CF, Persson MMA, Koenig S, Chanock RM, Lerner RA. A large array of human monoclonal antibodies to type 1 human immunodeficiency virus from combinatorial libraries of asymptomatic seropositive individuals. Proc Natl Acad Sci USA. 1991;88:10134–7.
Persson MAA, Caothien RH, Burton DR. Generation of diverse high-affinity human monoclonal antibodies by repertoire cloning. Proc Natl Acad Sci USA. 1991;88:2432–6.
Siegel DL. Phage-display tools for blood typing. Curr Hematol Rep. 2005;4:459–64.
Cines DB, Blanchette VS. Immune thrombocytopenic purpura. N Engl J Med. 2002;346:995–1008.
Cines DB, McKenzie SE, Siegel DL. Mechanisms of action of therapeutics in idiopathic thrombocytopenic purpura. J Pediatr Hematol Oncol. 2003;25:S52–6.
Roark JH, Bussel JB, Cines DB, Siegel DL. Genetic analysis of autoantibodies in ITP reveals evidence of clonal expansion and somatic mutation. Blood. 2002;100:1388–98.
Silverman GJ, Goodyear CS, Siegel DL. On the mechanism of Staphylococcal protein A immunomodulation. Transfusion. 2005;45:274–80.
Goodyear CS, Sugiyama F, Silverman GJ. Temporal and dose-dependent relationships between in vivo B cell receptor-targeted proliferation and deletion-induced by a microbial B cell toxin. J Immunol. 2006;176:2262–71.
Siegel DL, Loftus JC, McMillan R. Characterization of an ITP patient-derived anti-α2bβ3 monoclonal antibody that inhibits platelet aggregation. Blood. 2003;102:87a.
Zheng X, Majerus EM, Sadler JE. ADAMTS13 and TTP. Curr Opin Hematol. 2002;9:389–94.
Ostertag EM, Kacir S, Gulendram G, Ai J, Smith P, Zheng XL, et al. Cloning inhibitory ADAMTS13 monoclonal antibodies from a TTP patient using phage display. Transfusion. 2005;45:1a.
Siegel DL, Ostertag EM. Inhibitory ADAMTS13 monoclonal autoantibodies cloned from TTP patients show restricted gene usage, inhibit murine ADAMTS13, and are blocked by rabbit anti-idiotypic antibodies. Blood. 2006;108:31a.
Payne AS, Ishii K, Kacir S, Lin C, Li H, Hanakawa Y, et al. Genetic and functional characterization of human pemphigus vulgaris monoclonal autoantibodies isolated by phage display. J Clin Invest. 2005;115:888–99.
Payne AS, Siegel DL, Stanley JR. Targeting pemphigus autoantibodies through their heavy chain variable region genes. J Invest Dermatol. 2007;127:1681–91.
Ishii K, Lin C, Siegel DL, Stanley JR. Isolation of pathogenic monoclonal anti-desmoglein 1 human antibodies by phage display of pemphigus foliaceus autoantibodies. J Invest Dermatol. 2007;128:939–48.
Yamagami I, Ishii K, Payne AS, Kacir S, Amagai M, Siegel DL, et al. Pathogenic pemphigus foliaceus (PF) and vulgaris (PV) antibodies share a consensus amino acid motif in their CDR3 regions. J Invest Dermatol. 2008;128:S7.
Stanley JR, Lin C, Siegel DL. Novel method for eipdermal drug delivery with non-pathogenic monoclonal pemphigus antibodies. J Invest Dermatol. 2007;127:S39.
Siegel DL, Silberstein LE. Expression and characterization of recombinant anti-Rh(D) antibodies on filamentous phage: a model system for isolating human red blood cell antibodies by repertoire cloning. Blood. 1994;83:2334–44.
Siegel DL. Phage display-based molecular methods in immunohematology. Transfusion. 2007;47:89S–94S.
Siegel DL, Reid ME, Lee H, Blancher A. Production of large repertories of macaque mAbs to human RBCs using phage display. Transfusion. 1999;39:92S.
Acknowledgments
The author acknowledges funding from the National Institutes of Health (K08-HL02621, R01-HL61844, R01-AR48223, R01-AR052672, P50-HL54516, P50-HL54500, P50-HL81012, R41-HL73533, R42-HL73533, T32-HL07775, T32-AR07465), the National Blood Foundation, the Lupus Foundation, the March of Dimes Birth Defects Foundation, the BioAdvance Biotechnology Greenhouse Fund of Southeastern Pennsylvania, and the UPenn Institute for Translational Medicine and Therapeutics.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Siegel, D.L. Translational applications of antibody phage display. Immunol Res 42, 118–131 (2008). https://doi.org/10.1007/s12026-008-8044-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-008-8044-y